Answered step by step
Verified Expert Solution
Question
1 Approved Answer
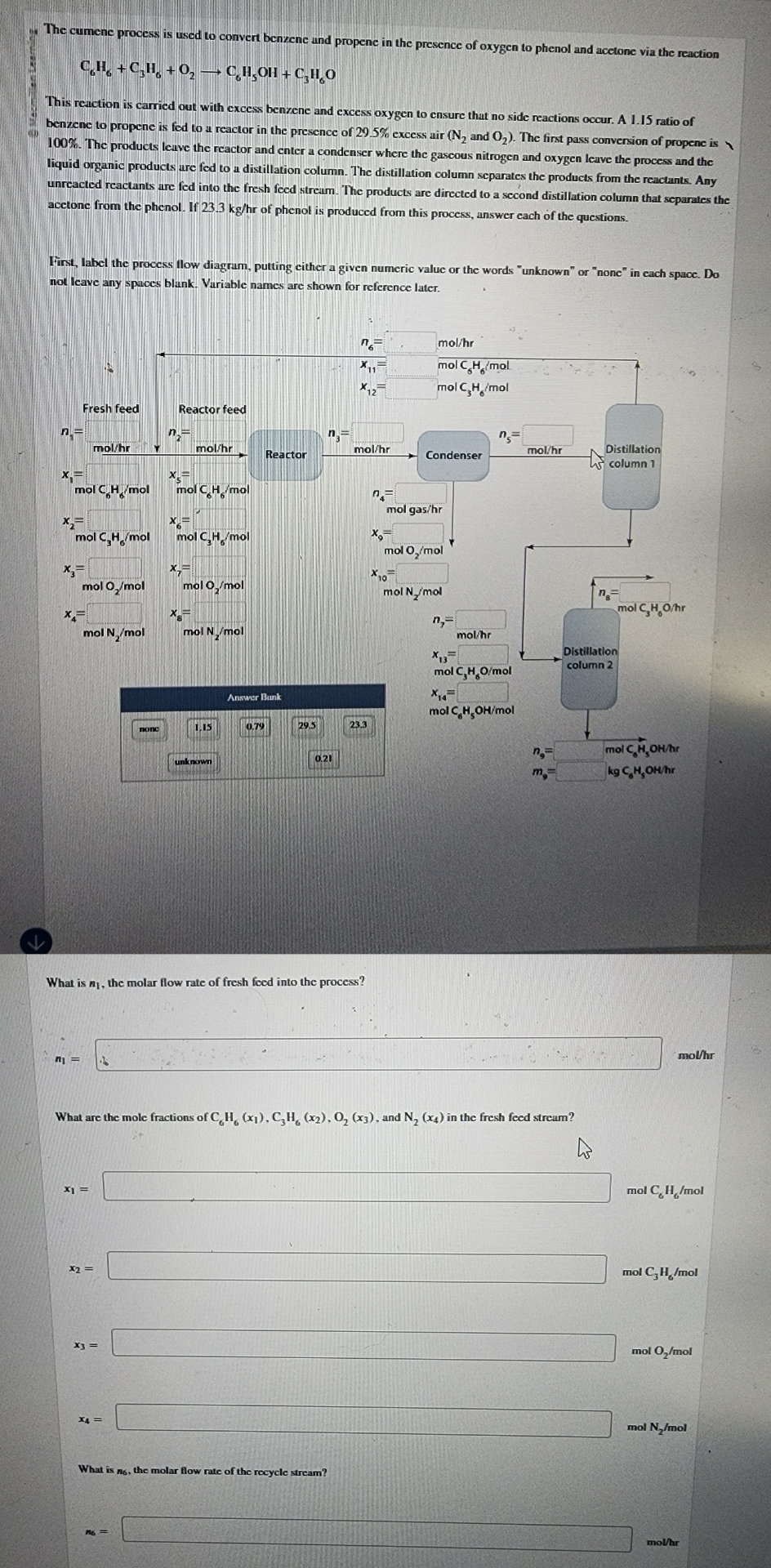
The cumene process is used to convert benzene and propene in the presence of oxygen to phenol and acetone via the reaction C 6 H
The cumene process is used to convert benzene and propene in the presence of oxygen to phenol and acetone via the reaction
This reaction is carried out with excess benrene and excess oxygen to ensure that no side reactions occur. A ratio of benzene to propene is fod to a reactor in the presence of excess air and The first pass conversion of propene is The products leave the reactor and enter a condenser where the gascous nitrogen and oxygen leave the process and the liquid organic products ure fed to a distillation column. The distillation column separates the products from the reactants. Any unreacted reactants are fed into the fresh feed stream. The products are directed to a second distillation column that separates the acetone from the phenol. If of phenol is produced from this process, answer each of the questions.
First, label the process flow diagrum, putting either a given numeric value or the words "unknown" or "none" in each space. Do not leave any spaces blank. Variable names are shown for reference later.
What is the molar flow rate of fresh foed into the process?
molhr
What are the mole fractions of and in the fresh feed stream?
What is the molar flow rate of the recycle stream?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


