Question
The data below is for the reaction sequence A-B-C-D Elementary step A,H/kJ mol E/kl mol A B 22 33 B1C -11 22 21D -33
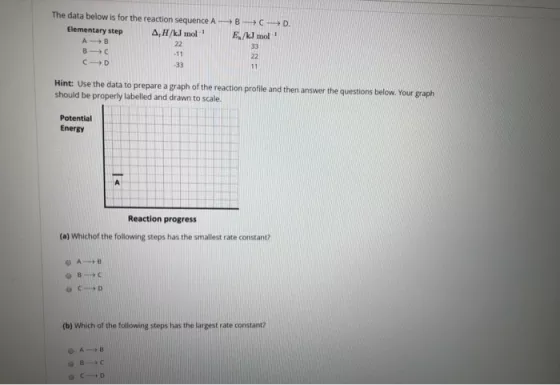
The data below is for the reaction sequence A-B-C-D Elementary step A,H/kJ mol E/kl mol A B 22 33 B1C -11 22 21D -33 11 Hint: Use the data to prepare a graph of the reaction profile and then answer the questions below. Your graph should be properly labelled and drawn to scale. Potential Energy Reaction progress (a) Which of the following steps has the smallest rate constant? 6810 2119 (b) Which of the following steps has the largest rate constant? DA 9216 |
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
a Since k AeEaRT Higner the Ea lesser is the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Law Principles and Practices
Authors: Arnold J. Goldman, William D. Sigismond
9th edition
1133586562, 978-1285632995, 1285632990, 978-1285675367, 978-1133586562
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



