Answered step by step
Verified Expert Solution
Question
1 Approved Answer
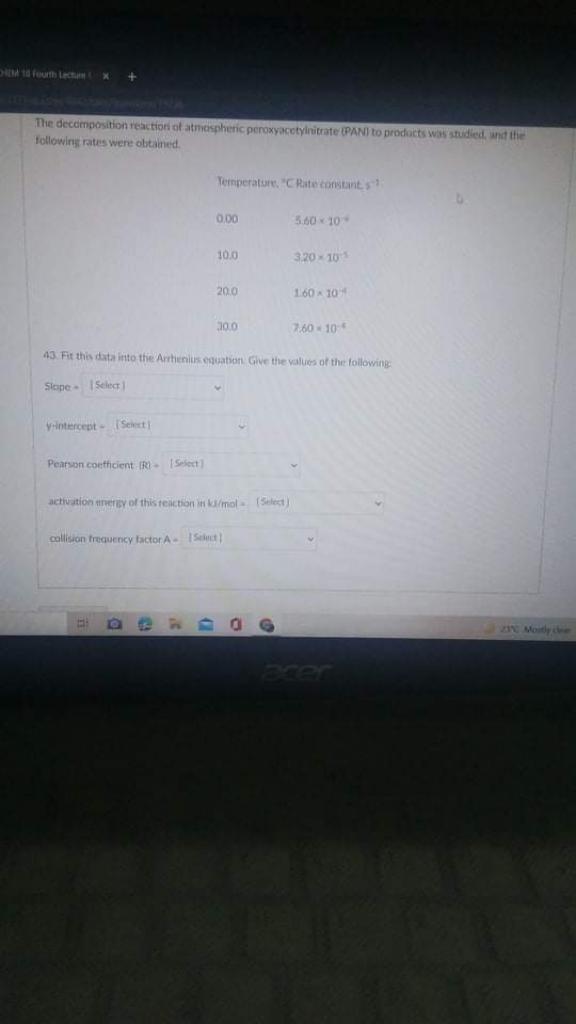
The decomposition reaction of atmospheric peroxyacetylnitrate (PAN) to products was studied and the following rates were obtained. Temperature Temperature Rate Constant 0.00 5.60 x 10
The decomposition reaction of atmospheric peroxyacetylnitrate (PAN) to products was studied and the following rates were obtained.
Temperature
| Temperature | Rate Constant |
| 0.00 | 5.60 x 10-4 |
| 10.00 | 3.20 x 10-5 |
| 20.00 | 1.60 x 10-4 |
| 30.00 | 7.60 x 10-4 |
Fit this data into the Arrhenius equation. Give the values of the following: Slope, y-intercept, Pearson coefficient (R), activation energy of this reaction in kJ/mol, collision frequency factor A
+ The decomposition reaction of atmospheric peroxyacetylnitrate PANI to products was studied, and the Following rates were obtained. Temperature, "Rate constants 0.00 56010 10.0 3.2010 20.0 160 x 10 300 7.6010 43. Fit this data into the Arxentis equation Give the values of the following: Slape Select intercept - Select Pearson coefficient R - Sfect activation etsy of this tection /mol (Stect collision frequency factor A Select Mody
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


