Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The density of perfectly crystalline polyethylene is c = 1000 kg m-3, and the density of perfectly amorphous polyethylene is a = 865 kg m-3.
The density of perfectly crystalline polyethylene is c = 1000 kg m-3, and the density of perfectly amorphous polyethylene is a = 865 kg m-3. (i) For a semi-crystalline material with mass density , show that the percent crystallinity (xc) can be calculated as: xc = a c a where c is the mass density of a 100% crystalline polymer. (ii) Calculate xc in a sample of linear polyethylene with s = 970 kg m-3 and a sample of branched polyethylene with = 917 kg m-3. (iii) Why do the two samples have considerably different crystalline content?
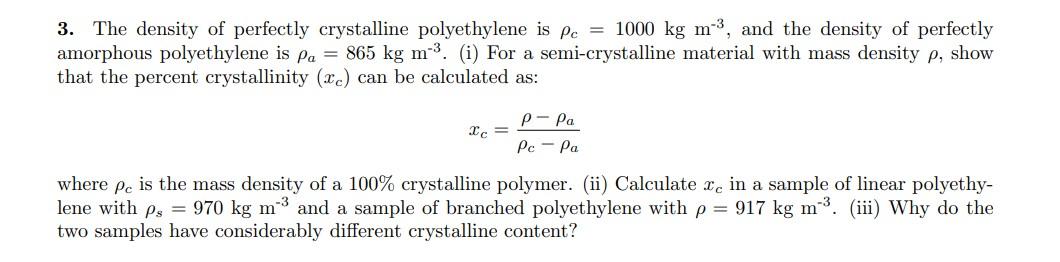
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


