Question
The distillation column shown below is used to separate compound A from compound B in an adiabatic fashion. A liquid mixture of A and B
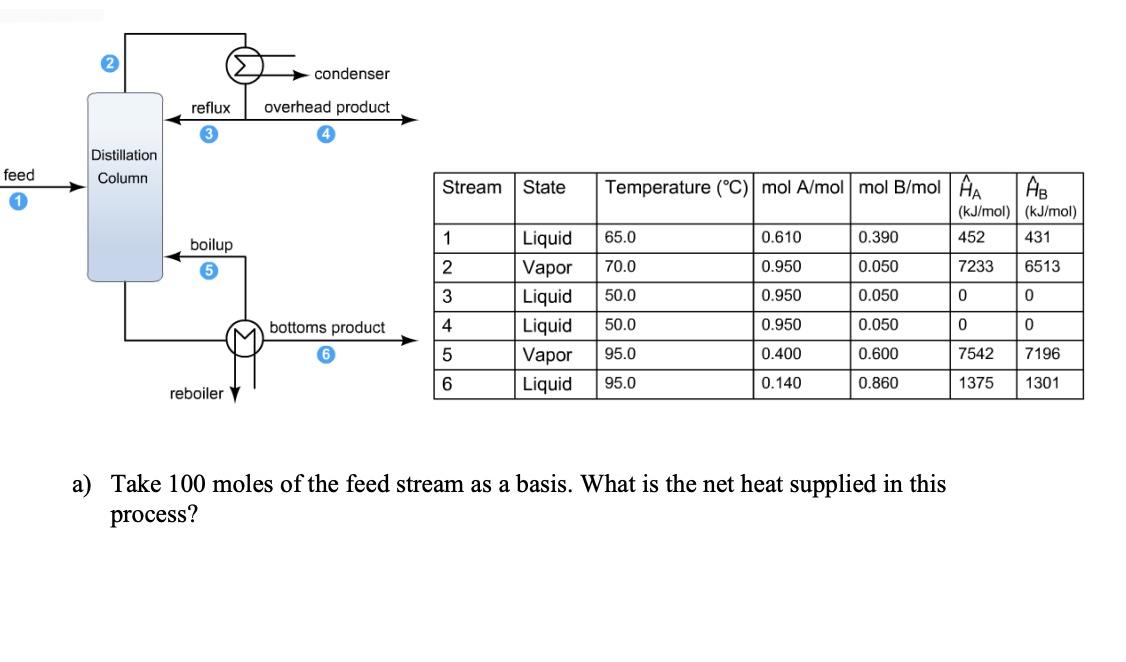
a) Take 100 moles of the feed stream as a basis. What is the net heat supplied in this process?
b) Again, taking 100 moles of the feed stream as the basis, what is the heat removed at the condenser (QC), and the heat inputted at the reboiler (QR)? (Make sure your answer for QC is negative, indicating that heat is removed by the condenser.)
c) Suppose now that instead of the condensed liquid from the top of column being split into two equal streams to form the reflux and the overhead product, it is split into two streams, with the reflux being 3 times the overhead product. As before, take a basis of 100 mol of feed and determine the net heating requirements for the process and the heat removed in the condenser and added in the reboiler.
feed Distillation Column reflux overhead product boilup condenser reboiler bottoms product Stream 1 2 3 4 5 6 State Temperature (C) mol A/mol mol B/mol A Liquid 65.0 Vapor 70.0 Liquid 50.0 50.0 Liquid Vapor 95.0 Liquid 95.0 0.610 0.950 0.950 0.950 0.400 0.140 0.390 0.050 0.050 0.050 0.600 0.860 (kJ/mol) (kJ/mol) 452 431 7233 6513 0 0 0 0 a) Take 100 moles of the feed stream as a basis. What is the net heat supplied in this process? 7542 1375 7196 1301
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


