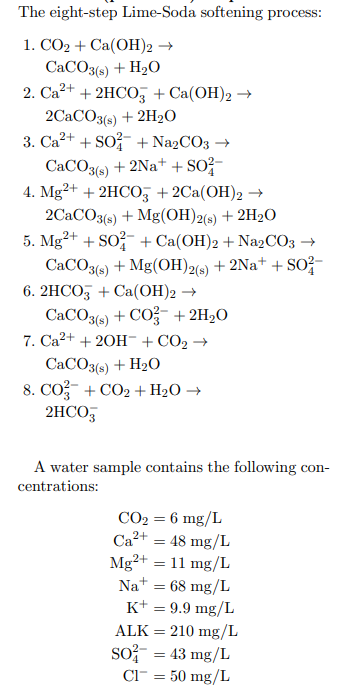
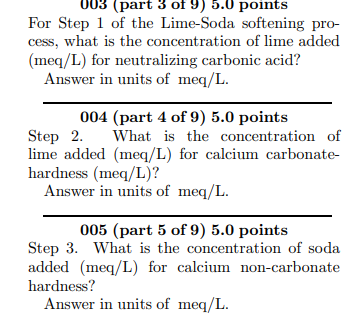
The eight-step Lime-Soda softening process: 1. CO2 + Ca(OH)2 CaCO3(s) + H2O 2. Ca2+ + 2HCO3 + Ca(OH)2 2CaCO3(s) + 2H20 3. Ca2+ + SO + Na2CO3 CaCO3(s) + 2Na+ + S02- 4. Mg2+ + 2HCO3 +2Ca(OH)2 2CaCO3(s) + Mg(OH)2(s) + 2H20 5. Mg2+ + S0 - + Ca(OH)2 + Na2CO3 CaCO3(s) + Mg(OH)2(g) + 2Na+ + S02 6. 2HCO3 + Ca(OH)2 CaCO3(s) + CO3 + 2H,0 7. Ca2+ + 2OH- + CO2 CaCO3(s) + H20 8. Co + CO2 + H20 2HCO3 A water sample contains the following con- centrations: CO2 = 6 mg/L Ca2+ = 48 mg/L Mg2+ = 11 mg/L Na+ = 68 mg/L K+ = 9.9 mg/L ALK = 210 mg/L so = 43 mg/L Cl" = 50 mg/L (part 3 of 9) 5.0 points For Step 1 of the Lime-Soda softening pro- cess, what is the concentration of lime added (meq/L) for neutralizing carbonic acid? Answer in units of meq/L. 004 (part 4 of 9) 5.0 points Step 2. What is the concentration of lime added (meq/L) for calcium carbonate- hardness (meq/L)? Answer in units of meq/L. 005 (part 5 of 9) 5.0 points Step 3. What is the concentration of soda added (meq/L) for calcium non-carbonate hardness? Answer in units of meq/L. The eight-step Lime-Soda softening process: 1. CO2 + Ca(OH)2 CaCO3(s) + H2O 2. Ca2+ + 2HCO3 + Ca(OH)2 2CaCO3(s) + 2H20 3. Ca2+ + SO + Na2CO3 CaCO3(s) + 2Na+ + S02- 4. Mg2+ + 2HCO3 +2Ca(OH)2 2CaCO3(s) + Mg(OH)2(s) + 2H20 5. Mg2+ + S0 - + Ca(OH)2 + Na2CO3 CaCO3(s) + Mg(OH)2(g) + 2Na+ + S02 6. 2HCO3 + Ca(OH)2 CaCO3(s) + CO3 + 2H,0 7. Ca2+ + 2OH- + CO2 CaCO3(s) + H20 8. Co + CO2 + H20 2HCO3 A water sample contains the following con- centrations: CO2 = 6 mg/L Ca2+ = 48 mg/L Mg2+ = 11 mg/L Na+ = 68 mg/L K+ = 9.9 mg/L ALK = 210 mg/L so = 43 mg/L Cl" = 50 mg/L (part 3 of 9) 5.0 points For Step 1 of the Lime-Soda softening pro- cess, what is the concentration of lime added (meq/L) for neutralizing carbonic acid? Answer in units of meq/L. 004 (part 4 of 9) 5.0 points Step 2. What is the concentration of lime added (meq/L) for calcium carbonate- hardness (meq/L)? Answer in units of meq/L. 005 (part 5 of 9) 5.0 points Step 3. What is the concentration of soda added (meq/L) for calcium non-carbonate hardness? Answer in units of meq/L








