Question
The electrochemical extraction of aluminum from bauxite ore involves (A) the reaction of AlO3 with coke (C) at a temperature > 2500 C. (B)
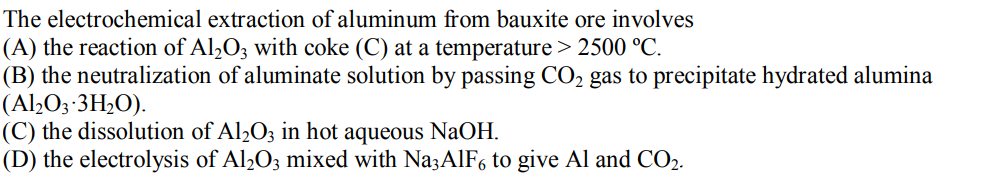
The electrochemical extraction of aluminum from bauxite ore involves (A) the reaction of AlO3 with coke (C) at a temperature > 2500 C. (B) the neutralization of aluminate solution by passing CO gas to precipitate hydrated alumina (AlO3-3HO). (C) the dissolution of Al2O3 in hot aqueous NaOH. (D) the electrolysis of Al2O3 mixed with Na3AlF6 to give Al and CO2.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Answer B t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Separation process principles
Authors: J. D. Seader
2nd Edition
471464805, 978-0471464808
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



