Question: The electron in a doubly ionized lithium atom (Li2+) is initially - in the ground state. If it absorbs a photon with energy Em it
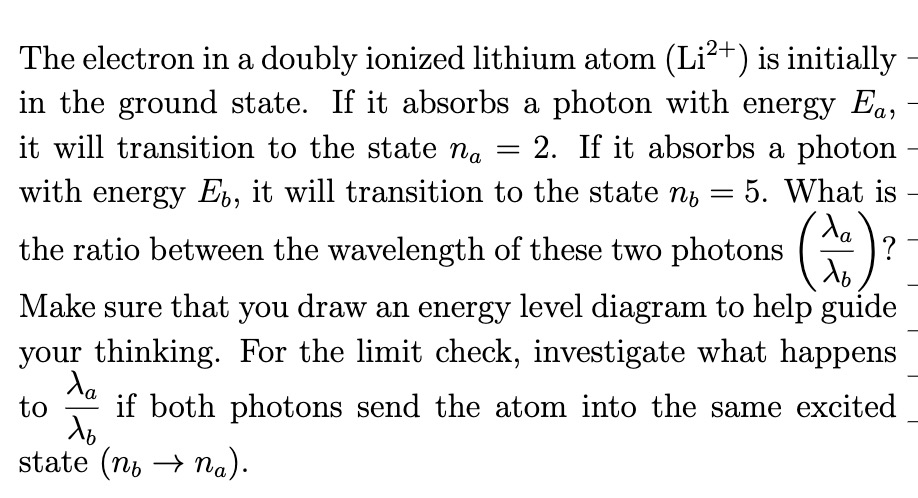

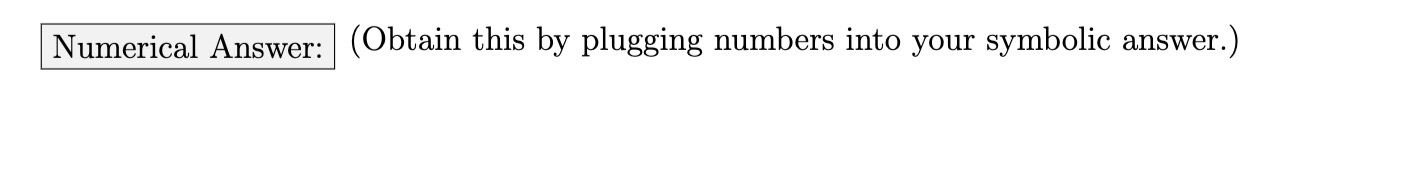
The electron in a doubly ionized lithium atom (Li2+) is initially - in the ground state. If it absorbs a photon with energy Em it will transition to the state n... = 2. If it absorbs a photon with energy Eb, it will transition to the state g, 2 5. What is A... the ratio between the wavelength of these two photons (T) ? ' b Make sure that you draw an energy level diagram to help guide _ your thinking. For the limit check, investigate what happens to Aa' if both photons send the atom into the same excited _ b state (71;, ) n6). A\" a) As n5 a> am what limit does [\\ approach? in b) Why does the result make physical sense? Numerical Answer: (Obtain this by plugging numbers into your symbolic answer.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


