Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The electrophilic aromatic substitution of isopropylbenzene with FeBr3, Br2 gives 1-bromo-4-isopropylbenzene. Complete the curved-arrow mechanism below, beginning with formation of the active brominating reagent. Remember
The electrophilic aromatic substitution of isopropylbenzene with FeBr3, Br2 gives 1-bromo-4-isopropylbenzene. Complete the curved-arrow mechanism below, beginning with formation of the active brominating reagent. Remember to include lone pairs and formal charges where appropriate. ONLY NEED HELP WITH STEPS 3 & 4. NOT SURE WHY, but I can't seem to get the ARROW PUSHING right.
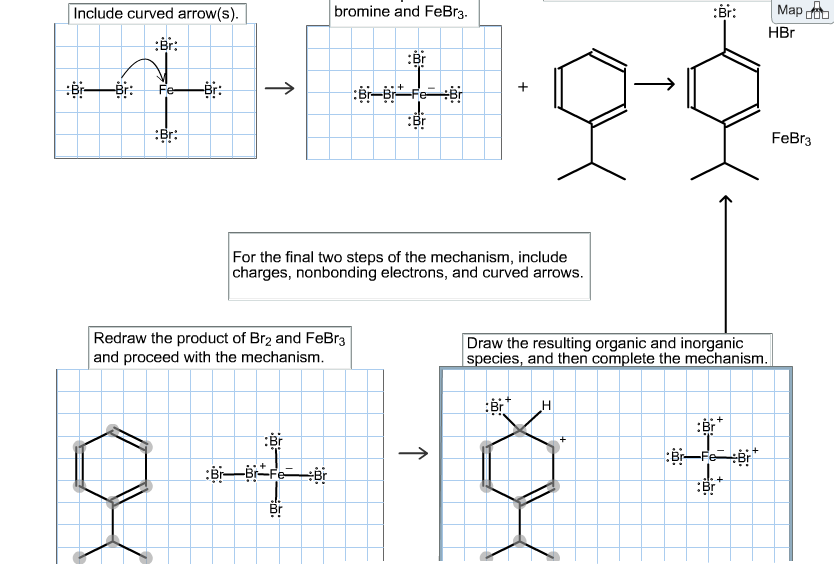
Include curved arrow(s). +++ Fe -Br: :Br: 0 :Br: bromine and FeBr3. Redraw the product of Br2 and FeBr3 and proceed with the mechanism. Br Br-Fe Br Br :Br Br-Br FeBr For the final two steps of the mechanism, include charges, nonbonding electrons, and curved arrows. :Br + H Draw the resulting organic and inorganic species, and then complete the mechanism. X :Br: Map duu HBr :Br Br-FeBr + :Br FeBr3
Step by Step Solution
★★★★★
3.45 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


