Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The elementary irreversible organic gas-phase reaction A + B +2 C is carried out adiabatically in a flow reactor. An equal molar feed in A
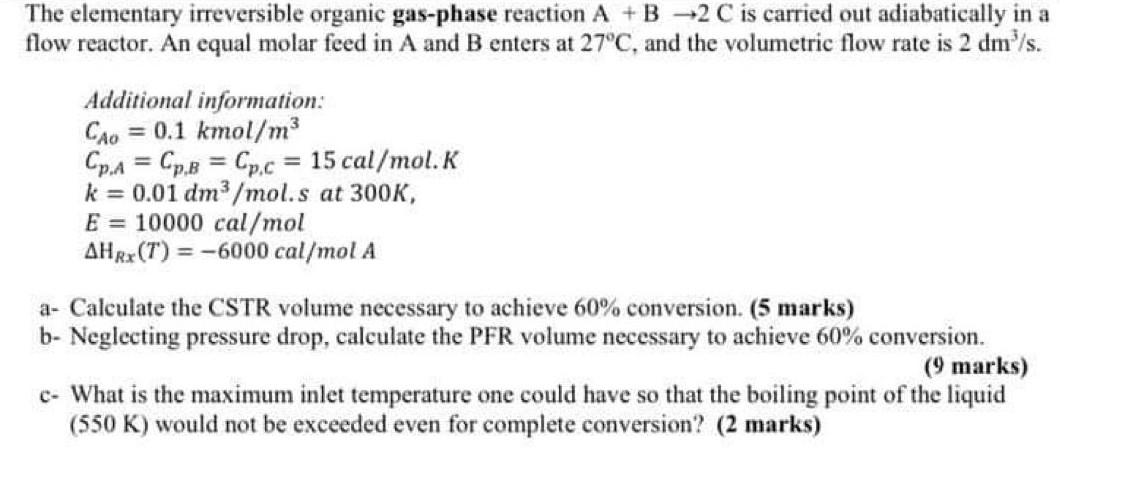
The elementary irreversible organic gas-phase reaction A + B +2 C is carried out adiabatically in a flow reactor. An equal molar feed in A and B enters at 27C, and the volumetric flow rate is 2 dm's. Additional information: CAO = 0.1 kmol/m3 Cp.a = Cp.B = Cpc = 15 cal/mol.K k = 0.01 dm3/mol.s at 300K, E = 10000 cal/mol AHRX(T) = -6000 cal/mol A a- Calculate the CSTR volume necessary to achieve 60% conversion. (5 marks) b- Neglecting pressure drop, calculate the PFR volume necessary to achieve 60% conversion. (9 marks) c- What is the maximum inlet temperature one could have so that the boiling point of the liquid (550 K) would not be exceeded even for complete conversion? (2 marks)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


