Answered step by step
Verified Expert Solution
Question
1 Approved Answer
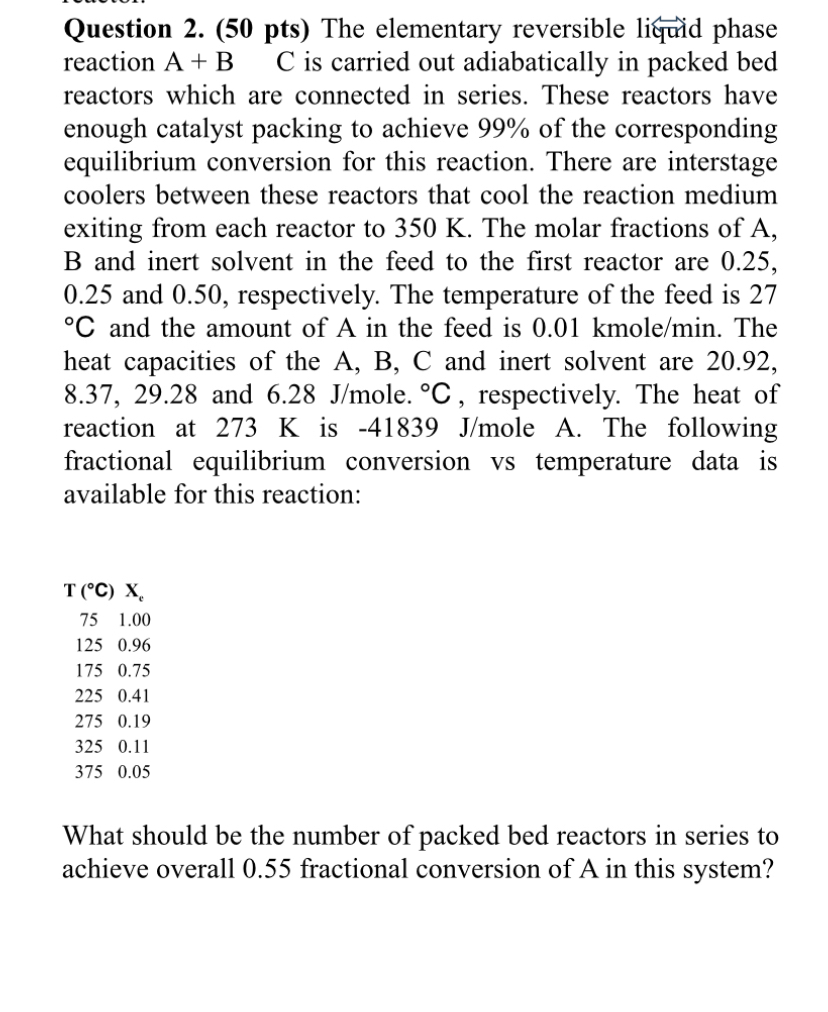
The elementary reversible li&nid phase reaction A + B C is carried out adiabatically in packed bed reactors which are connected in series. These reactors
The elementary reversible li&nid phase reaction A B C is carried out adiabatically in packed bed reactors which are connected in series. These reactors have enough catalyst packing to achieve of the corresponding equilibrium conversion for this reaction. There are interstage coolers between these reactors that cool the reaction medium exiting from each reactor to The molar fractions of and inert solvent in the feed to the first reactor are and respectively. The temperature of the feed is and the amount of in the feed is kmol The heat capacities of the and inert solvent are and ole. respectively. The heat of reaction at is oleA. The following fractional equilibrium conversion vs temperature data is available for this reaction:What should be the number of packed bed reactors in series to achieve overall fractional conversion of A in this system?I want a graphical solution to this question.Do not forget that it is an exo reaction.Also it should be shown as both xe and adiabatic in the graph
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


