Question
2. A solution of CuSO4 was prepared, followed by a series of serial dilutions. Absorbance measurements were taken with max = 645 nm in
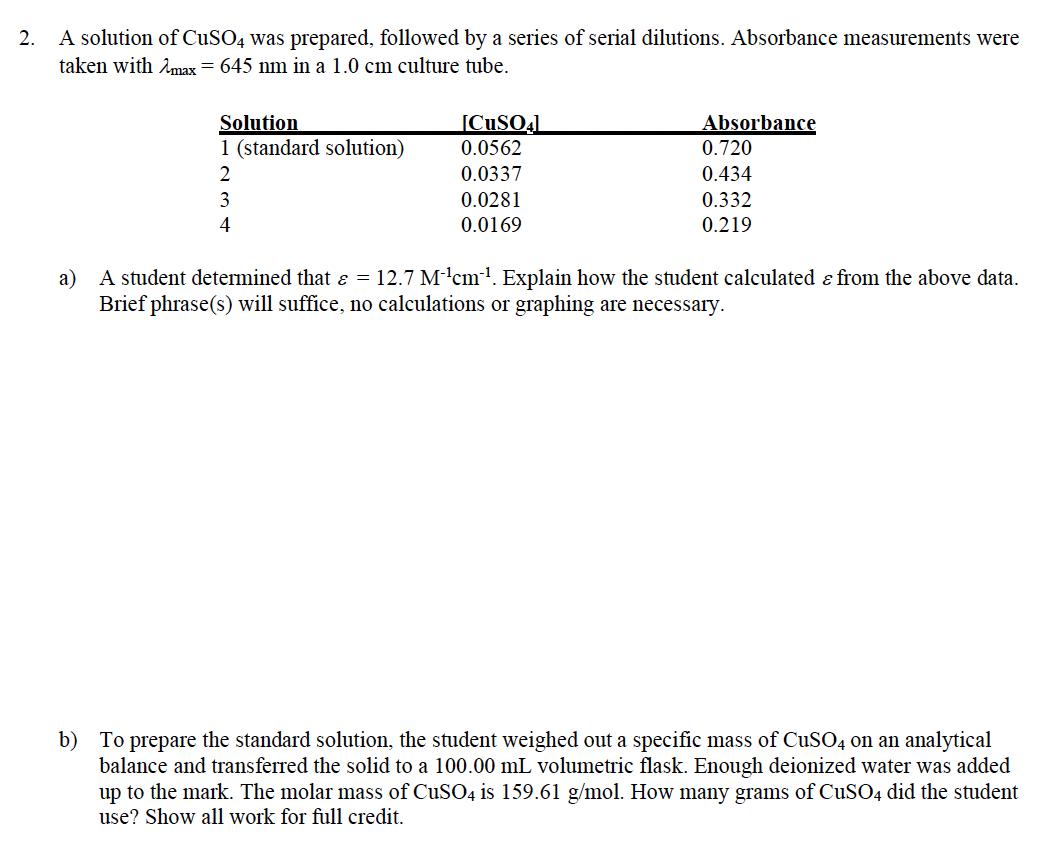
2. A solution of CuSO4 was prepared, followed by a series of serial dilutions. Absorbance measurements were taken with max = 645 nm in a 1.0 cm culture tube. Solution [CuSO4L 1 (standard solution) 0.0562 23 0.0337 0.0281 0.0169 Absorbance 0.720 0.434 0.332 0.219 4 a) A student determined that & = 12.7 Mcm. Explain how the student calculated & from the above data. Brief phrase(s) will suffice, no calculations or graphing are necessary. b) To prepare the standard solution, the student weighed out a specific mass of CuSO4 on an analytical balance and transferred the solid to a 100.00 mL volumetric flask. Enough deionized water was added up to the mark. The molar mass of CuSO4 is 159.61 g/mol. How many grams of CuSO4 did the student use? Show all work for full credit.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



