Question
The equation of state for a mixture has exactly the same form but depend on mixing parameters. Empirical mixing rules relate mixture parameters to
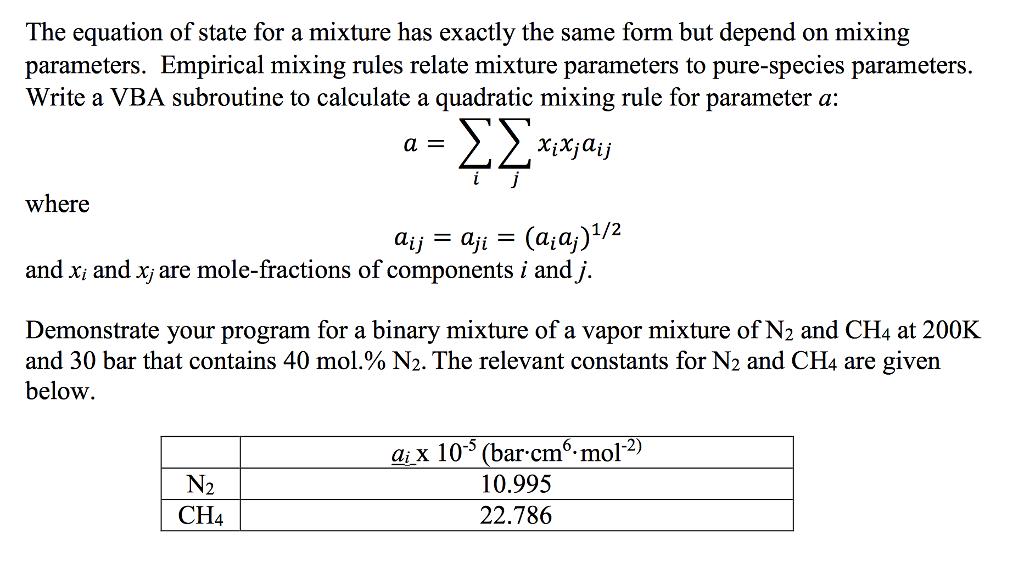
The equation of state for a mixture has exactly the same form but depend on mixing parameters. Empirical mixing rules relate mixture parameters to pure-species parameters. Write a VBA subroutine to calculate a quadratic mixing rule for parameter a: where a = Xixjaij = aij = aji (ajaj) 1/2 and x; and x; are mole-fractions of components i and j. Demonstrate your program for a binary mixture of a vapor mixture of N2 and CH4 at 200K and 30 bar that contains 40 mol.% N2. The relevant constants for N2 and CH4 are given below. aix 105 (bar-cm-mol-2) N2 CH4 10.995 22.786
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics Fundamentals And Engineering Applications
Authors: William C. Reynolds, Piero Colonna
1st Edition
0521862736, 9780521862738
Students also viewed these Programming questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



