Question
The equilibrium constant, K, for the following reaction is 1.2010-2 at 500 K. PCI5(g) =PCl;(g)+ Cl2(g) An equilibrium mixture of the three gases in
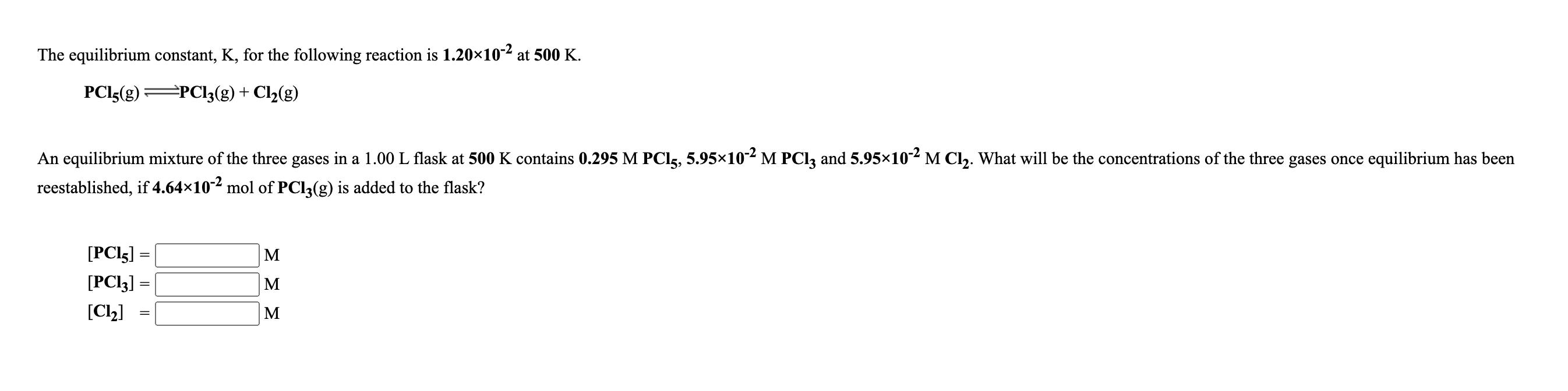
The equilibrium constant, K, for the following reaction is 1.2010-2 at 500 K. PCI5(g) =PCl;(g)+ Cl2(g) An equilibrium mixture of the three gases in a 1.00 L flask at 500 K contains 0.295 M PCI5, 5.9510- M PCI3 and 5.9510 M Cl. What will be the concentrations of the three gases once equilibrium has been reestablished, if 4.64x10-2 mol of PCI3(g) is added to the flask? [PCI5] M [PCI3] = M [C2] M
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Given The equilibrium constant K for the following reaction i...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



