Answered step by step
Verified Expert Solution
Question
1 Approved Answer
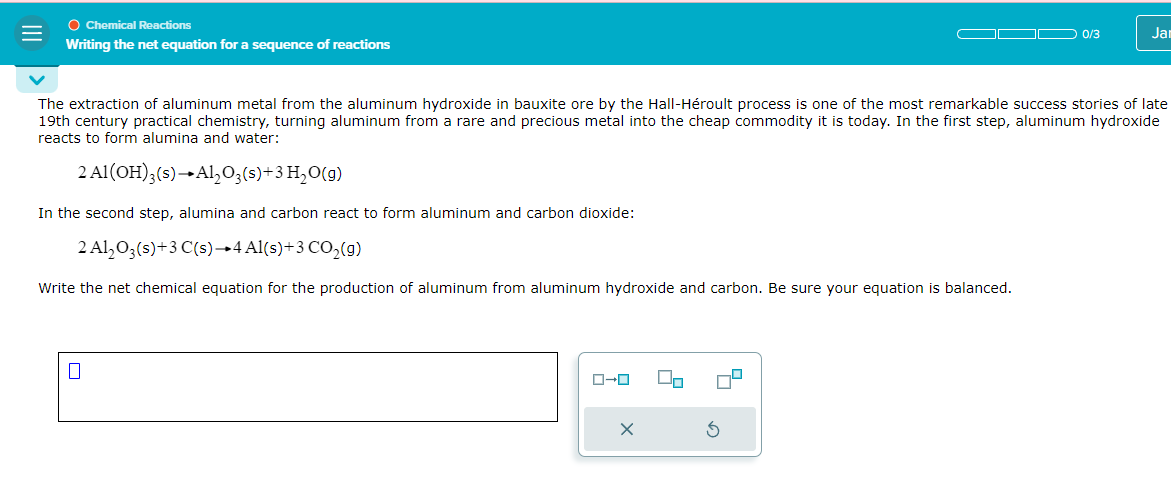
The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall - H roult process is one of the most remarkable
The extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the HallHroult process is one of the most remarkable success stories of late
th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide
reacts to form alumina and water:
In the second step, alumina and carbon react to form aluminum and carbon dioxide:
Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


