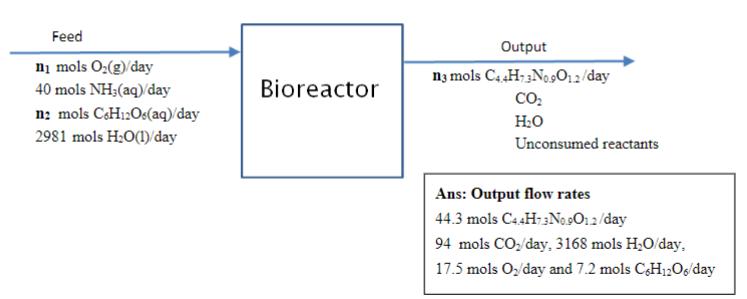
Question
The feed to the bioreactor is oxygen gas and an aqueous solution of ammonia and glucose. The water (not including the ammonia and glucose dissolved
The feed to the bioreactor is oxygen gas and an aqueous solution of ammonia and glucose. The
water (not including the ammonia and glucose dissolved in it) in the feed aqueous solution enters
the bioreactor at a flowrate of 2982 mols H2 O/day (see Figure below). The Ammonia in the feed
is completely consumed in the bioreactor. The glucose is 15 % in excess with respect to
ammonia. The oxygen gas is 25% in excess with respect to ammonia. Please help solve for the molar flow rates of all species leaving the bioreactor. Answers are shown in the photo so if yours don't match up they are wrong.
C6 H12 O6 + 1.45 O2 + 0.83 NH3 --> 0.92 C4.4 H7.3 N0.9 O1.2 + 3.88 H2 O + 1.95 CO2
Feed ni mols O(g)/day 40 mols NH3(aq)/day n: mols C6H12O6(aq)/day 2981 mols HO(1)/day Bioreactor Output n3 mols C4.4H73N0.9012/day CO HO Unconsumed reactants Ans: Output flow rates 44.3 mols C4.4H73No.9O12/day 94 mols CO/day, 3168 mols HO/day. 17.5 mols Oy/day and 7.2 mols C6H12O6/day
Step by Step Solution
3.39 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
To solve for the molar flow rates of all species leaving the bioreactor we need to apply stoichiomet...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


