Answered step by step
Verified Expert Solution
Question
1 Approved Answer
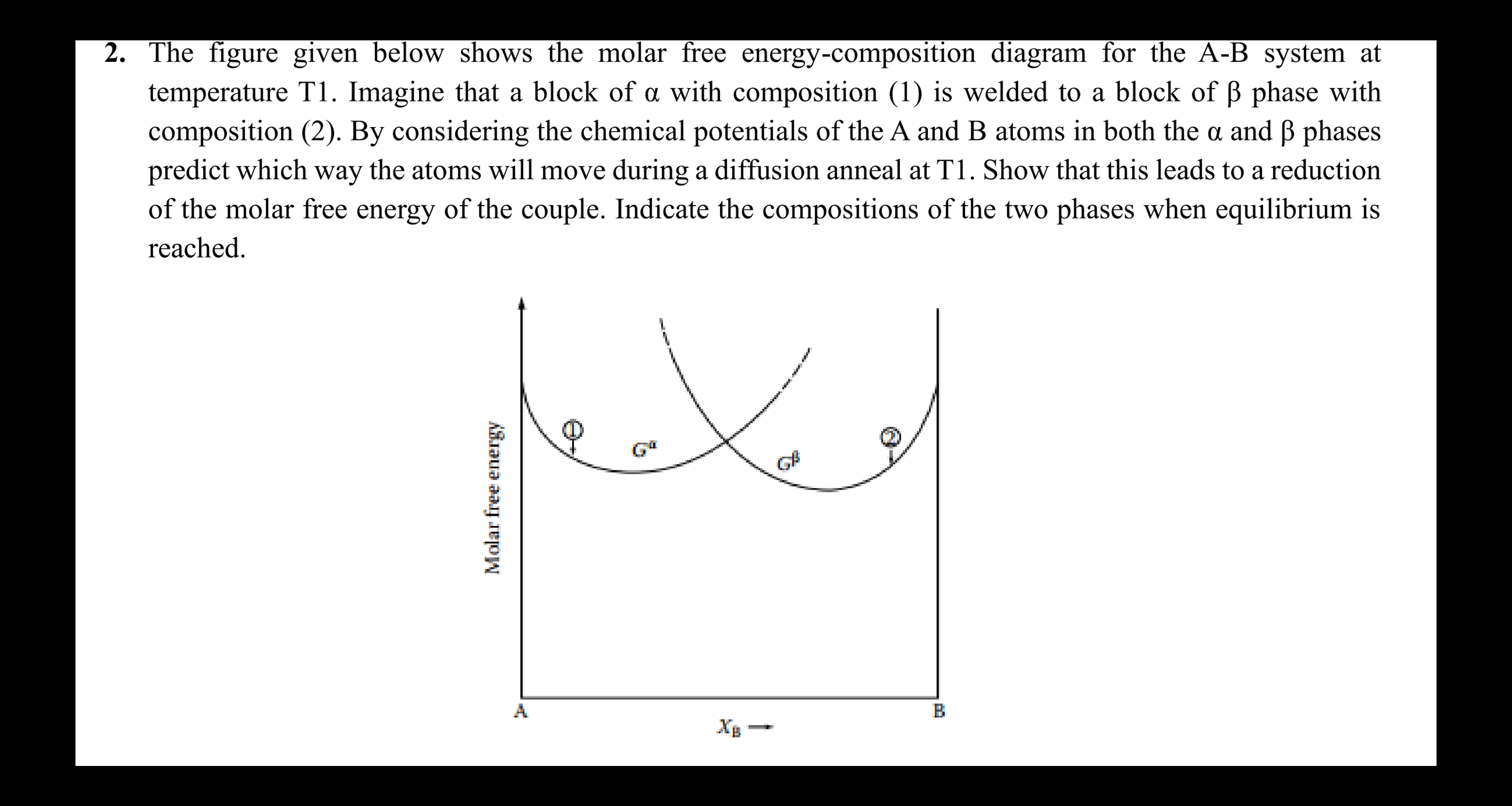
The figure given below shows the molar free energy - composition diagram for the A - B system at temperature T 1 . Imagine that
The figure given below shows the molar free energycomposition diagram for the AB system at
temperature T Imagine that a block of with composition is welded to a block of phase with
composition By considering the chemical potentials of the A and B atoms in both the and phases
predict which way the atoms will move during a diffusion anneal at T Show that this leads to a reduction
of the molar free energy of the couple. Indicate the compositions of the two phases when equilibrium is
reached.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


