Answered step by step
Verified Expert Solution
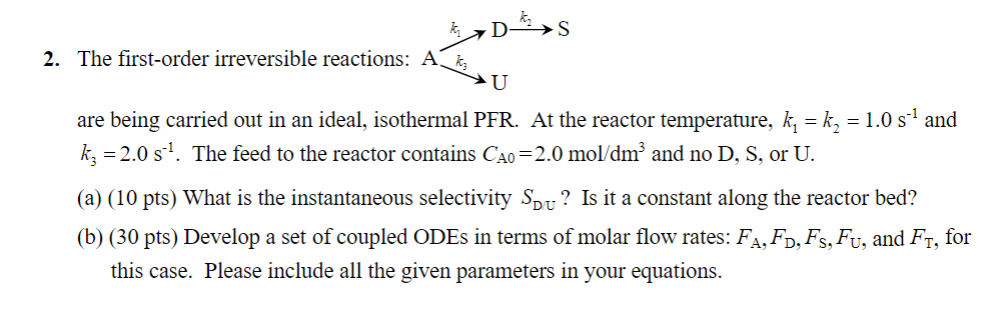
Question
1 Approved Answer
The first - order irreversible reactions: are being carried out in an ideal, isothermal PFR . At the reactor temperature, k 1 = k 2
The firstorder irreversible reactions:
are being carried out in an ideal, isothermal PFR At the reactor temperature, and
The feed to the reactor contains and no or
a pts What is the instantaneous selectivity Is it a constant along the reactor bed?
b pts Develop a set of coupled ODEs in terms of molar flow rates: and for
this case. Please include all the given parameters in your equations.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


