Question
The following data apply to the binary system of A and B: Melting point of pure A = 1050C Melting point of pure B
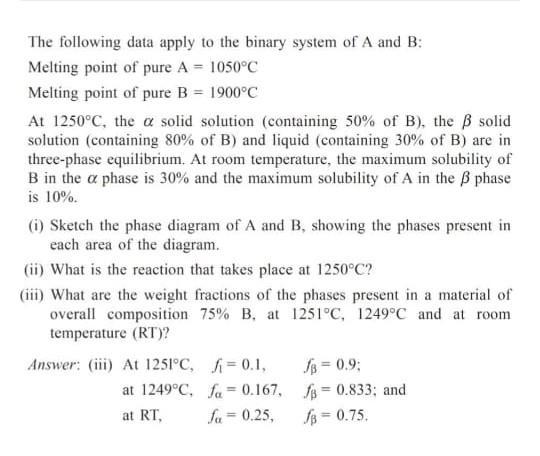
The following data apply to the binary system of A and B: Melting point of pure A = 1050C Melting point of pure B = 1900C At 1250C, the a solid solution (containing 50% of B), the B solid solution (containing 80% of B) and liquid (containing 30% of B) are in three-phase equilibrium. At room temperature, the maximum solubility of B in the a phase is 30% and the maximum solubility of A in the B phase is 10%. (i) Sketch the phase diagram of A and B, showing the phases present in each area of the diagram. (ii) What is the reaction that takes place at 1250C? (iii) What are the weight fractions of the phases present in a material of overall composition 75% B, at 1251C, 1249C and at room temperature (RT)? Answer: (ii) At 1251C, =0.1, fa = 0.9; at 1249C, fa= 0.167, f = 0.833; and %3D at RT, fa = 0.25, fs = 0.75. %3D
Step by Step Solution
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Reporting And Analysis Using Financial Accounting Information
Authors: Charles H Gibson
12th Edition
1439080607, 978-1439080603
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



