Answered step by step
Verified Expert Solution
Question
1 Approved Answer
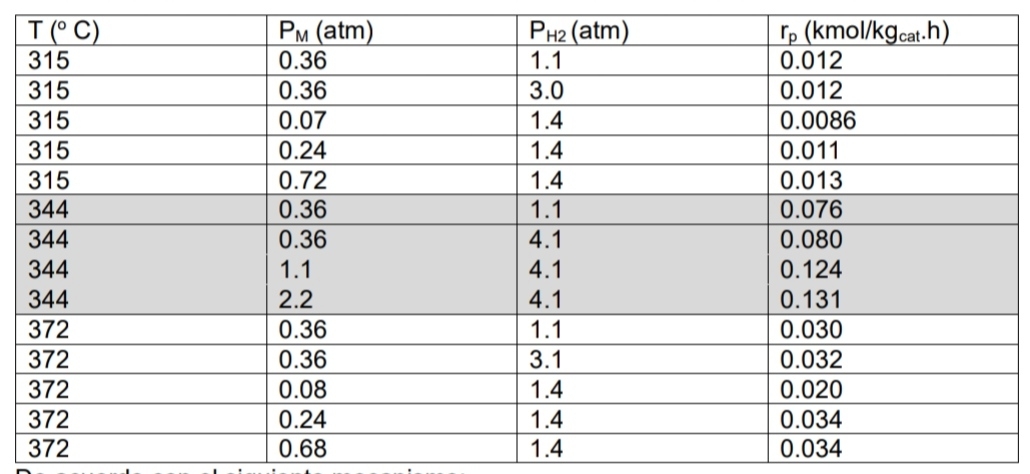
The following data was obtained by Sinfelt and company for the dehydrogenation of methyl cyclohexane to toluene. Furthermore, they discovered that the partial pressure of
The following data was obtained by Sinfelt and company for the dehydrogenation of methyl cyclohexane to toluene. Furthermore, they discovered that the partial pressure of toluene has no effect on the rate of reaction. According to the following mechanism: MSMS
MSRS
RS THS
Where M methyl cyclohexane, T toluene and H diatomic hydrogen. Develop the velocity equation for the following cases: a Adsorption is the limiting step b Surface reaction is the limiting step c Which of the two equations obtained in parts a and b presents a best fit based on the experimental data in the table? Present the value of the kinetic parameters with their units and the coefficient of determination R for each equation. d Based on the kinetic parameters of the equation that obtained the best fit, determine the activation energy.
table
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


