Question
The following equation denotes the surface tension ( ) for an anionic surfactant with a 14-carbon chain in pure water at 25C with the concentration
The following equation denotes the surface tension (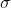 ) for an anionic surfactant with a 14-carbon chain in pure water at 25C with the concentration C in units of mol/L:
) for an anionic surfactant with a 14-carbon chain in pure water at 25C with the concentration C in units of mol/L:
a) Determine the pC20 value, where C20 is the concentration required to reduce the surface tension of pure solvent or solution by 20 mNm1. The pC20 value is also known as "surfactant efficiency"
b) If the surface tension of a solution of this surfactnat with a concentration of C = 0.25 mol/L is measured to be 29.0 mN/m, estimate the CMC of the surfactant.
c) If the surfactant above were exchanged for the 16-carbon member of the same series, describe how the surface tension data would change and give a numerical estimate of the new pC20 value.
o = 72.2 15.9 In (1 + (2.764 x 104) C) o = 72.2 15.9 In (1 + (2.764 x 104) C)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


