Question
The following figure shows EH versus pH diagram for chromium in water at 25 o C. a) From the figure, determine the dominant form of
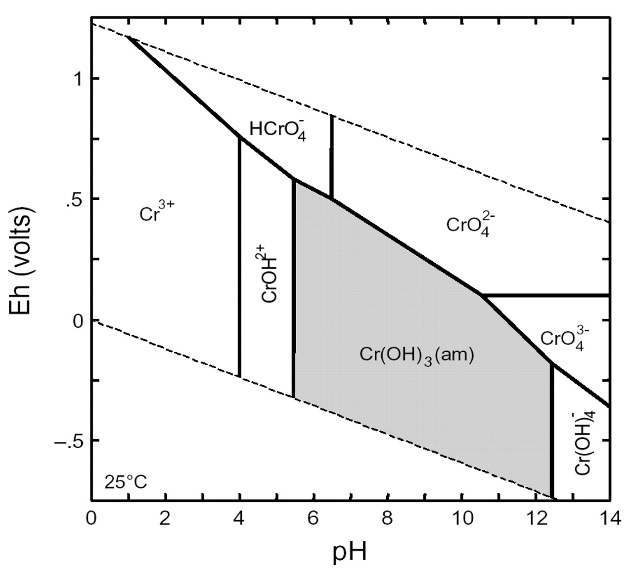
The following figure shows EH versus pH diagram for chromium in water at 25oC. a) From the figure, determine the dominant form of chromium at pH = 7 and pe = 12. b) Develop a balanced oxidation-reduction reaction for the conversion of CrO42- to Cr(OH)3(s) in the presence of elemental iron, which reacts to form Fe2+. Show the relevant half reactions, identify the oxidation and reduction half reactions, note the oxidation state of each redox-active element, and give the balanced net reaction. c) For a system with CrT = 10-5 M, use Figure to help determine the concentrations of the species CrO42- and Cr3+ at pH = 7.0 and EH = 100 mV. Calculate the concentration of total dissolved Cr under these conditions.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


