Question
The following is a diagram of energy states and transitions in the hydrogen atom. ENERGY 1 2 3 4 5 Match each arrow with
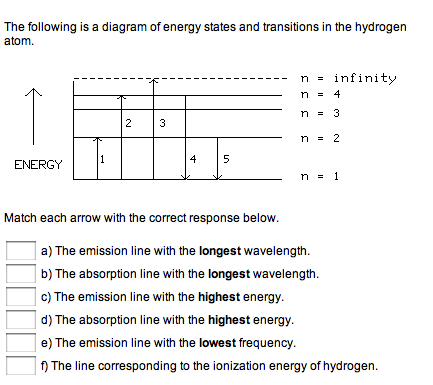
The following is a diagram of energy states and transitions in the hydrogen atom. ENERGY 1 2 3 4 5 Match each arrow with the correct response below. n n = 4 n = 3 n = 2 II infinity n = 1 a) The emission line with the longest wavelength. b) The absorption line with the longest wavelength. c) The emission line with the highest energy. d) The absorption line with the highest energy. e) The emission line with the lowest frequency. f) The line corresponding to the ionization energy of hydrogen.
Step by Step Solution
3.56 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Answer Suice E hc 1 Ex L D a for longest wavelength energ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Statistics Picturing The World
Authors: Ron Larson, Betsy Farber
6th Edition
0321911210, 978-0321911216
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



