Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following Laboratory results have been obtained from an air quality analysis: Particulate Matter: 77 ug/m3. SOxi 0.05 ppm NOx: 0.12 ppm CO: 6.0 ppm
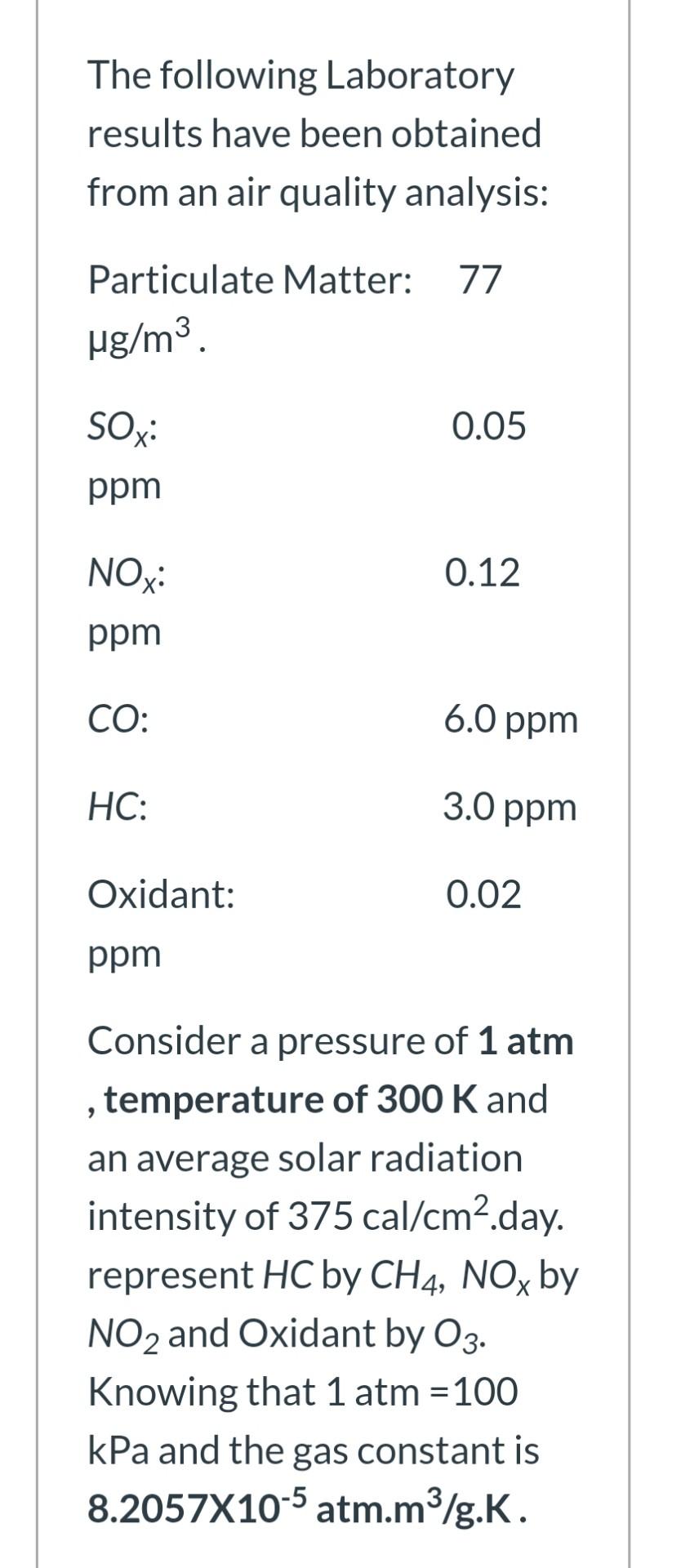
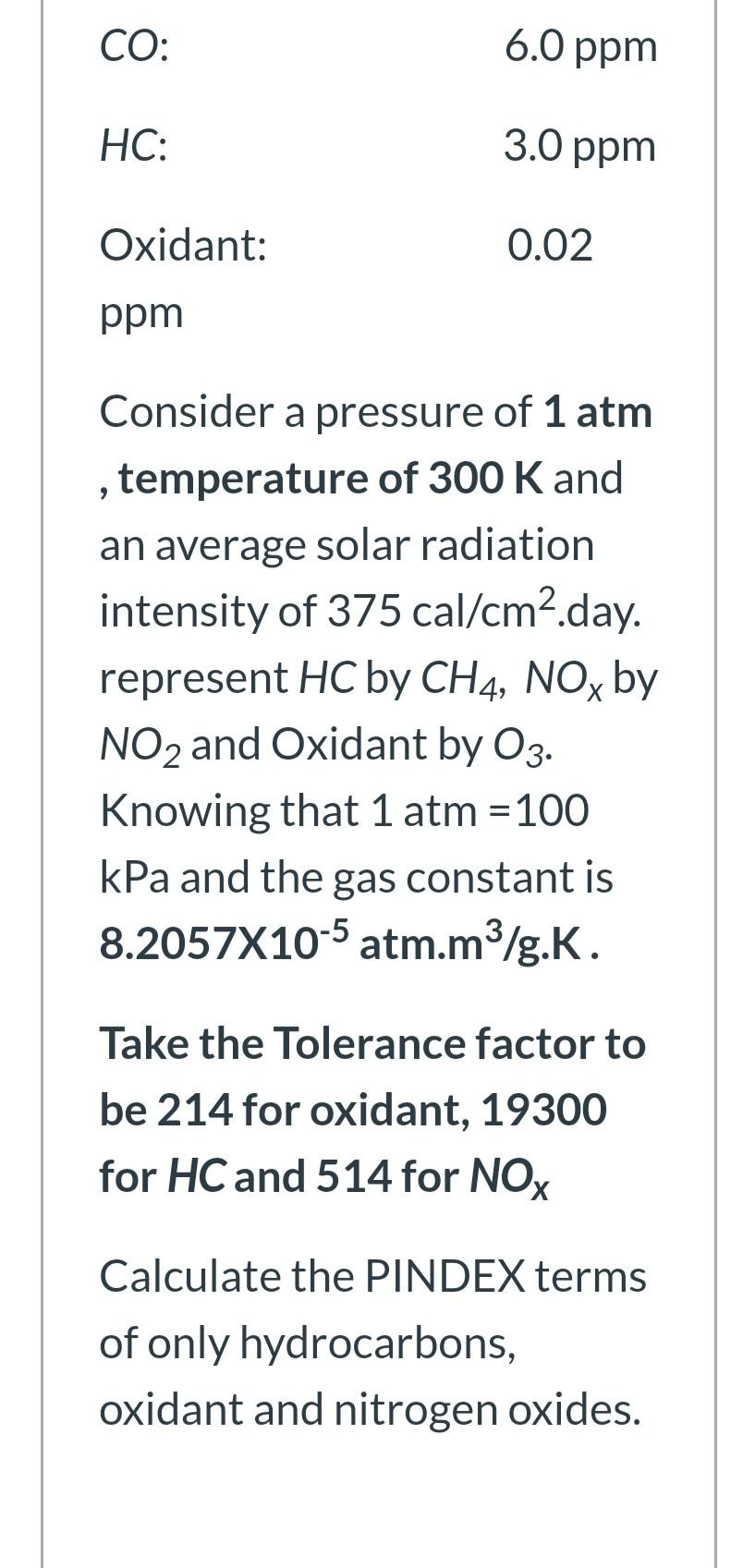
The following Laboratory results have been obtained from an air quality analysis: Particulate Matter: 77 ug/m3. SOxi 0.05 ppm NOx: 0.12 ppm CO: 6.0 ppm HC: : 3.0 ppm Oxidant: 0.02 ppm 9 Consider a pressure of 1 atm temperature of 300 K and an average solar radiation intensity of 375 cal/cm2.day. represent HC by CH4, NOx by NO2 and Oxidant by 03. Knowing that 1 atm = 100 kPa and the gas constant is 8.2057X10-5 atm.m3/g.K. CO: 6.0 ppm HC: 3.0 ppm Oxidant: 0.02 ppm > Consider a pressure of 1 atm temperature of 300 K and an average solar radiation intensity of 375 cal/cm2.day. represent HC by CH4, NOx by NO2 and Oxidant by O3. Knowing that 1 atm = 100 kPa and the gas constant is 8.2057X10-5 atm.m3/g.K. Take the Tolerance factor to be 214 for oxidant, 19300 for HC and 514 for NOx Calculate the PINDEX terms of only hydrocarbons, oxidant and nitrogen oxides
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


