Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following liquid-phase reactions were carried out in a CSTR at 325 K. kLA = 7.0 min- - B+C k0 = 3.0 dm mol?-min
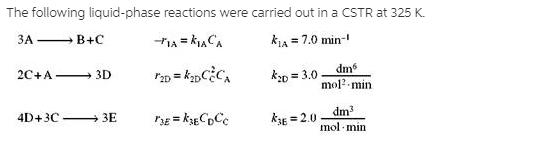

The following liquid-phase reactions were carried out in a CSTR at 325 K. kLA = 7.0 min- - B+C k0 = 3.0 dm mol?-min 2C+A- 3D dm mol min 4D+3C 3E kE = 2.0 !! The concentrations measured inside the reactor were CA = 0.10, CB = 0.93, CC = 0.51 , and CD = 0.049 all in mol/dm3. %3D (a) What are 1A, 12A, and 13A? (1A = - 0.7 mol/dm3 min) (b) What are 1B , 2B , and r3B? (c) What are 1C, r2C, and r3C? (1C = 0.23 mol/dm3 min) (d) What are r1D, 12D , and 13D? (e) What are 1E 2E, and r3E? (f) What are the net rates of formation of A, B, C, D, and E? (g) The entering volumetric flow rate is 100 dm3/min and the entering concentration of A is 3M. What is the CSTR reactor volume? (h) Write a Polymath program to calculate the exit concentrations when the volume is given as 600 dm3 (1) PFR. Now assume the reactions take place in the gas phase. Use the preceding data to plot the molar flow rates as a function of PFR volume. The pressure drop parameter is 0.001 dm-3, the total concentration entering the reactor is 0.2 mol/dm3, and uo = 100 dm3/min. What are D/E and SC/D? () Membrane Reactor. Repeat (i) when species C diffuses out of a membrane reactor and the transport coefficient, kC, is 10 min-1. Compare your results with part (1).
Step by Step Solution
★★★★★
3.30 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


