Question
The following multiple elementary liquid phase reactions take place in a continuous stirred tank reactor (CSTR): where D is the desired product and U is
The following multiple elementary liquid phase reactions take place in a continuous stirred tank reactor (CSTR): 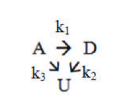 where D is the desired product and U is the undesired one. The reactor inlet consists of a solution containing only A at a concentration of CA0. (a) Formulate mass balances for all 3 components of the reaction in the CSTR. (b) Derive the equations for the concentrations of the three reaction components, CA, CD, and CU, as a function of space-time, , the three kinetic constants, and the concentration CA0. (c) Prove that the optimal space time (), for which the concentration of D is maximized, is given by the following equation:
where D is the desired product and U is the undesired one. The reactor inlet consists of a solution containing only A at a concentration of CA0. (a) Formulate mass balances for all 3 components of the reaction in the CSTR. (b) Derive the equations for the concentrations of the three reaction components, CA, CD, and CU, as a function of space-time, , the three kinetic constants, and the concentration CA0. (c) Prove that the optimal space time (), for which the concentration of D is maximized, is given by the following equation: 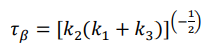 (d) Derive an equation for converting A to optimal space time
(d) Derive an equation for converting A to optimal space time
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


