Question
The following series reactions take place in the liquid phase: A --> B --> C The rate of destruction of A is -A =
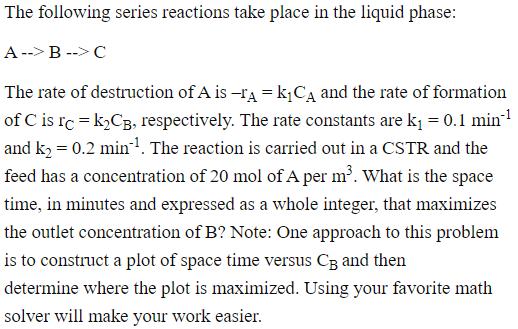
The following series reactions take place in the liquid phase: A --> B --> C The rate of destruction of A is -A = kCA and the rate of formation of C is rc = K2CB, respectively. The rate constants are k = 0.1 min and k = 0.2 min. The reaction is carried out in a CSTR and the feed has a concentration of 20 mol of A per m. What is the space time, in minutes and expressed as a whole integer, that maximizes the outlet concentration of B? Note: One approach to this problem is to construct a plot of space time versus CB and then determine where the plot is maximized. Using your favorite math solver will make your work easier.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



