Complete a reaction table (in millimoles) for the net reaction resulting when 41.2 mL of 0.0850 M AgNO3 and 32.3 mL of 0.170 M
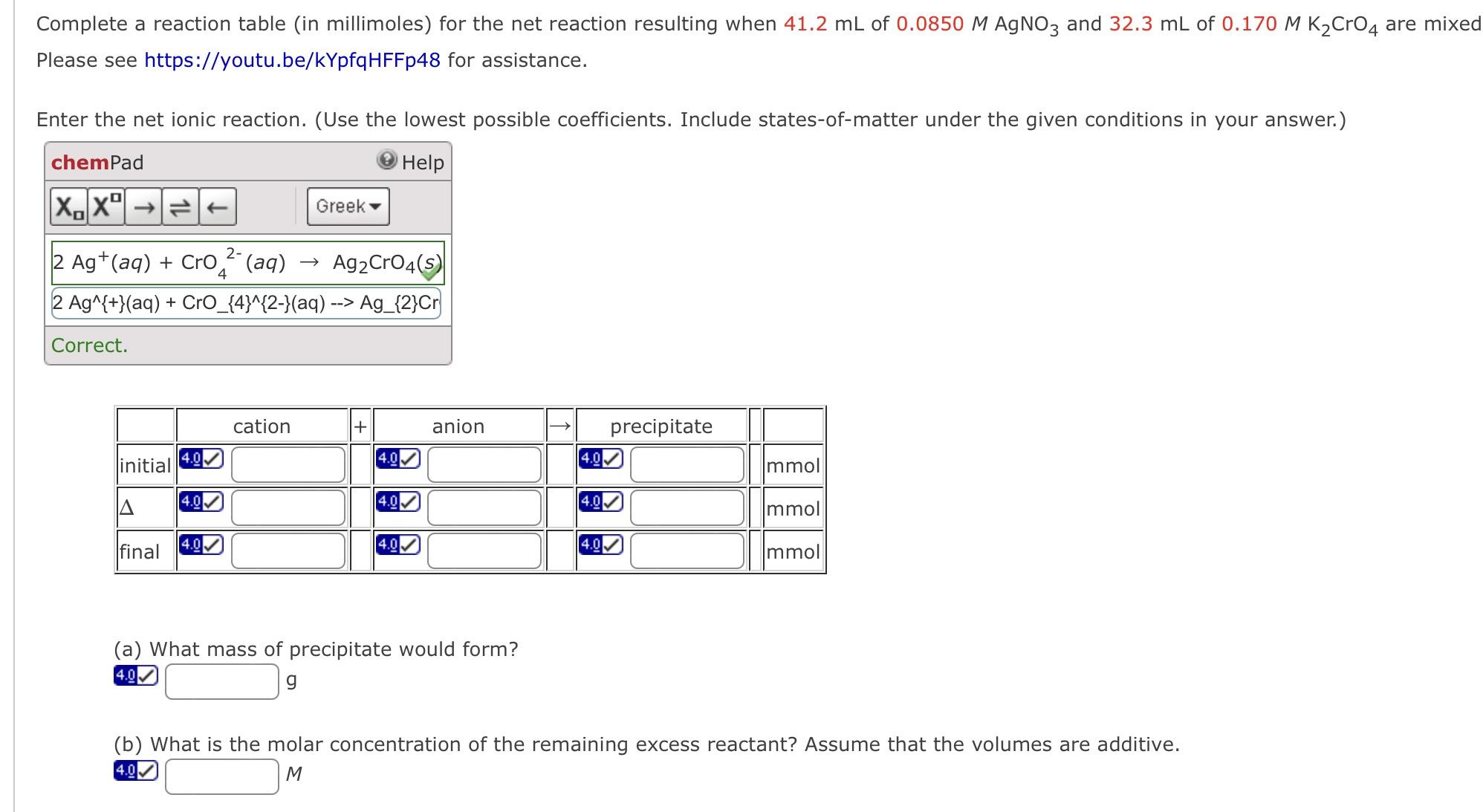
Complete a reaction table (in millimoles) for the net reaction resulting when 41.2 mL of 0.0850 M AgNO3 and 32.3 mL of 0.170 M K2CrO4 are mixed Please see https://youtu.be/kYpfqHFFp48 for assistance. Enter the net ionic reaction. (Use the lowest possible coefficients. Include states-of-matter under the given conditions in your answer.) chemPad XX Help Greek 2- 4 2 Ag+(aq) + Cro(aq) Ag2CrO4(s) 2 Ag^{+}(aq) + CrO_{4}^{2-}(aq) --> Ag_{2}Cr Correct. cation + anion precipitate 4.0 4.0 4.0 initial Immol 4.0 4.0 4.0 mmol final 4.0 4.0 4.0 mmol (a) What mass of precipitate would form? 4.0 g (b) What is the molar concentration of the remaining excess reactant? Assume that the volumes are additive. M 4.0
Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


