Answered step by step
Verified Expert Solution
Question
1 Approved Answer
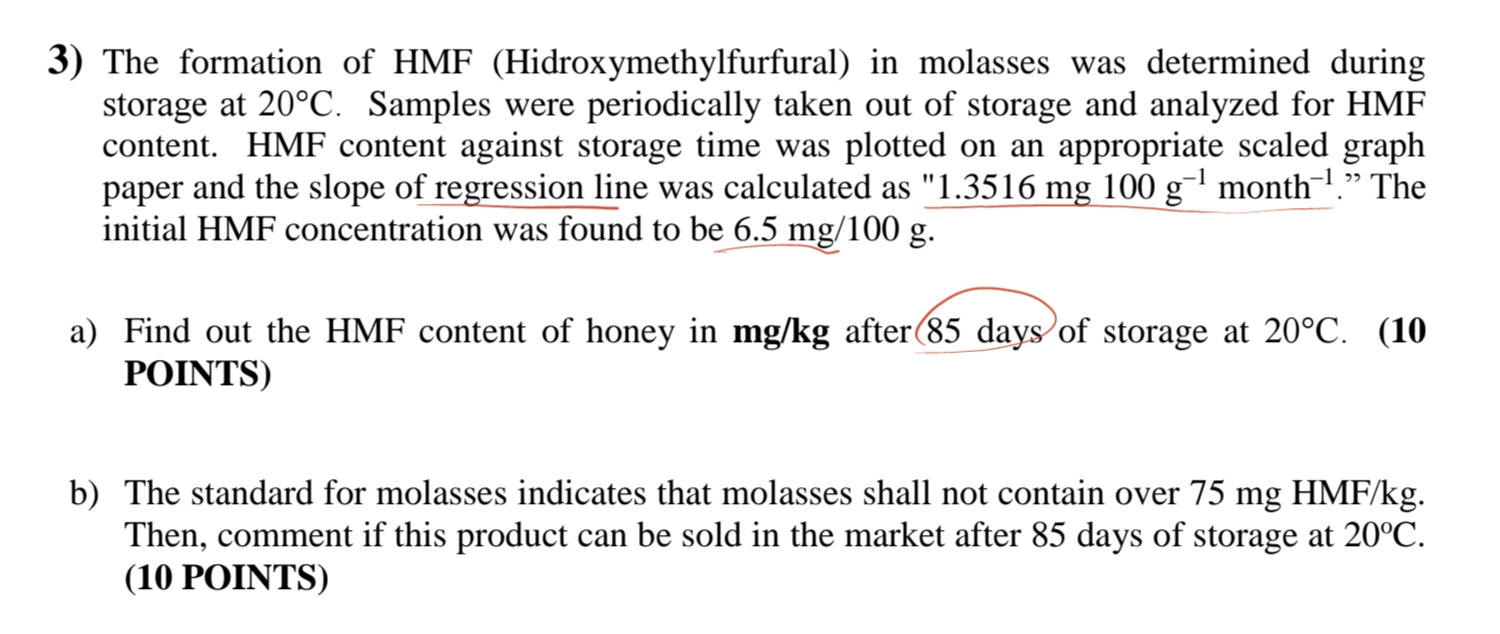
The formation of HMF ( Hidroxymethylfurfural ) in molasses was determined during storage at 2 0 C . Samples were periodically taken out of storage
The formation of HMF Hidroxymethylfurfural in molasses was determined during storage at Samples were periodically taken out of storage and analyzed for HMF content. HMF content against storage time was plotted on an appropriate scaled graph initial HMF concentration was found to be
a Find out the HMF content of honey in after days of storage at POINTS
b The standard for molasses indicates that molasses shall not contain over Then, comment if this product can be sold in the market after days of storage at POINTSThe formation of HMF Hidroxymethylfurfural in molasses was determined during
storage at deg C Samples were periodically taken out of storage and analyzed for HMF
content. HMF content against storage time was plotted on an appropriate scaled graph
paper and the slope of regression line was calculated as mg g month The
initial HMF concentration was found to be mg g
a Find out the HMF content of honey in mgkg after days of storage at deg C
POINTS
b The standard for molasses indicates that molasses shall not contain over mg HMFkg
Then, comment if this product can be sold in the market after days of storage at oC
POINTS
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


