Question
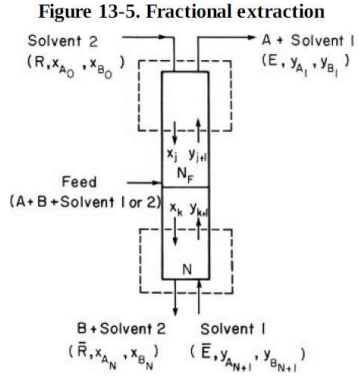
The fractional extraction system shown in Figure 13-5 is separating abietic acid from other acids. Solvent 1, heptane, enters at = 1000 kg/h and is
The fractional extraction system shown in Figure 13-5 is separating abietic acid from other acids. Solvent 1, heptane, enters at = 1000 kg/h and is pure. Solvent 2, methylcellosolve + 10% water, is pure and has a flow rate of R = 2500 kg/h. Feed is 5 wt % abietic acid in solvent 2 and flows at 1 kg/h. There are only traces of other acids in the feed. We desire to recover 95% of the abietic acid in the bottom raffinate stream. Feed is on stage 6. Assume that the solvents are completely immiscible and that the system can be considered to be very dilute. Equilibrium data are given in Table 13-3. Find N.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


