Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The frictionless piston-cylinder device shown in the figure has a saturated mixture of water at 105C and quality x = 0.85 occupying a volume
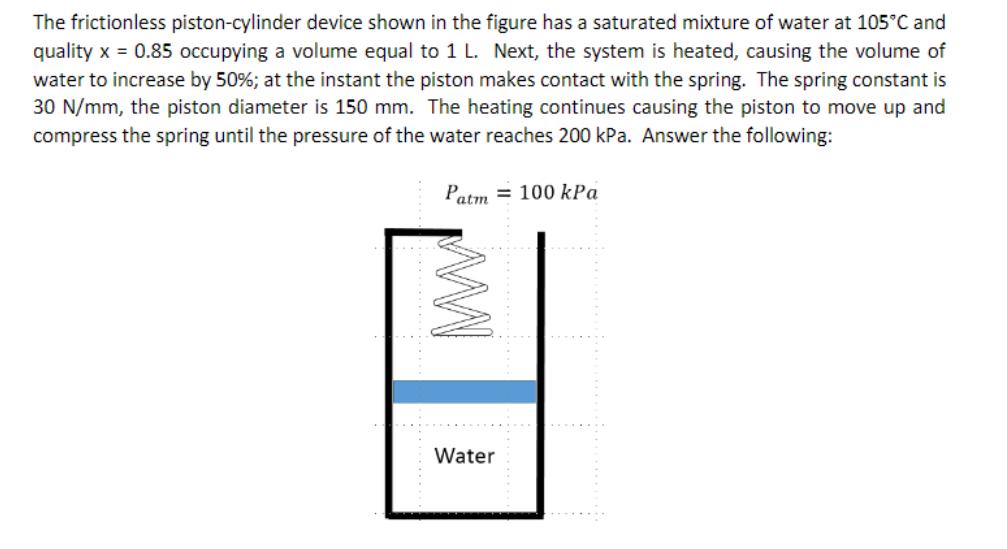

The frictionless piston-cylinder device shown in the figure has a saturated mixture of water at 105C and quality x = 0.85 occupying a volume equal to 1 L. Next, the system is heated, causing the volume of water to increase by 50%; at the instant the piston makes contact with the spring. The spring constant is 30 N/mm, the piston diameter is 150 mm. The heating continues causing the piston to move up and compress the spring until the pressure of the water reaches 200 kPa. Answer the following: Patm 100 kPa WWW Water a. Determine the mass of water in the piston-cylinder device, in kg. Show your equations in literal form before starting any computation; include units in your input values for partial computations. Express your answer with three significant figures. b. Determine the mass of the piston, in kg, with two decimal places. To receive credit for your solution please show a free body diagram and use it as part of your solution. c. For better interpretation of the physics undergoing in the water draw a system boundary and consider two sequential processes. 1-2: from the initial state until the piston makes initial contact with the spring; and 2-3: for the water expansion process until the pressure reaches 200 kPa. On a P-v diagram, represent the process 1-2-3; clearly show the saturation lines and pressure (kPa) and specific volume (m/kg) values. In order to receive credit for this question you are required to draw a free body diagram for the piston for processes 1-2 and 2-3, separately. d. What is the final temperature of the water, T3, in 'C? e. Compute the work per unit mass done by the water on the piston during process 1-2-3, in kJ/kg. f. Compute the heat that needs to be supplied to the device per unit mass of water, in kJ/lg. Assume that the device is perfectly insulated, and no heat leaves the system.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


