Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The gas generated by the reaction between zirconium and steam is released into the nuclear reactor headspace and can react explosively with another gas found
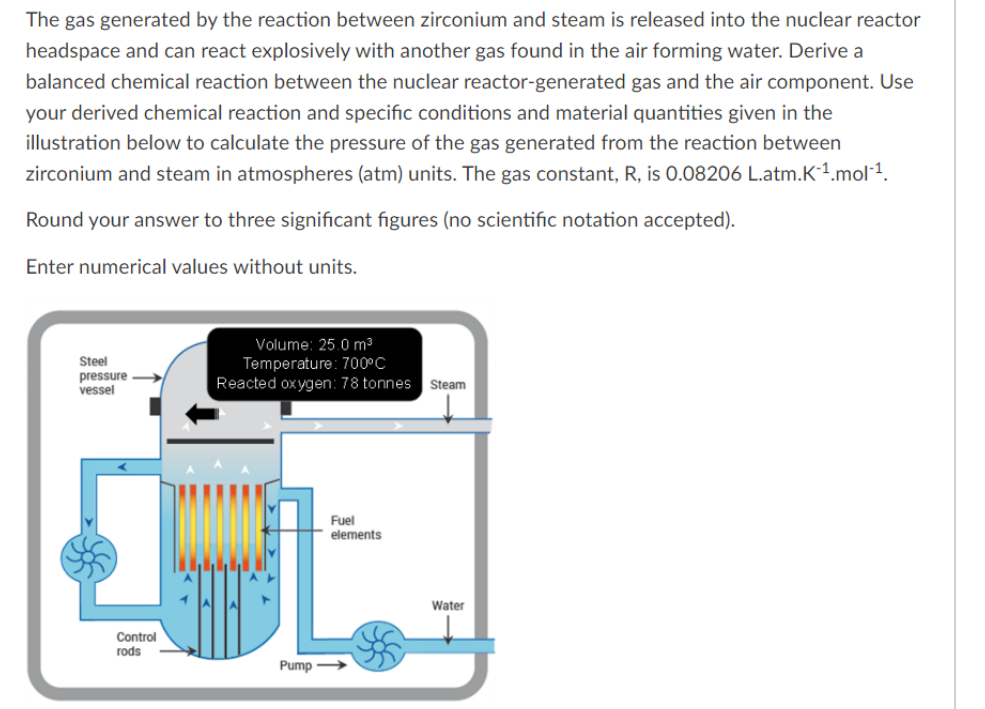 The gas generated by the reaction between zirconium and steam is released into the nuclear reactor headspace and can react explosively with another gas found in the air forming water. Derive a balanced chemical reaction between the nuclear reactor-generated gas and the air component. Use your derived chemical reaction and specific conditions and material quantities given in the illustration below to calculate the pressure of the gas generated from the reaction between zirconium and steam in atmospheres (atm) units. The gas constant, R, is 0.08206L.atmK1mol1. Round your answer to three significant figures (no scientific notation accepted). Enter numerical values without units. The gas generated by the reaction between zirconium and steam is released into the nuclear reactor headspace and can react explosively with another gas found in the air forming water. Derive a balanced chemical reaction between the nuclear reactor-generated gas and the air component. Use your derived chemical reaction and specific conditions and material quantities given in the illustration below to calculate the pressure of the gas generated from the reaction between zirconium and steam in atmospheres (atm) units. The gas constant, R, is 0.08206L.atmK1mol1. Round your answer to three significant figures (no scientific notation accepted). Enter numerical values without units
The gas generated by the reaction between zirconium and steam is released into the nuclear reactor headspace and can react explosively with another gas found in the air forming water. Derive a balanced chemical reaction between the nuclear reactor-generated gas and the air component. Use your derived chemical reaction and specific conditions and material quantities given in the illustration below to calculate the pressure of the gas generated from the reaction between zirconium and steam in atmospheres (atm) units. The gas constant, R, is 0.08206L.atmK1mol1. Round your answer to three significant figures (no scientific notation accepted). Enter numerical values without units. The gas generated by the reaction between zirconium and steam is released into the nuclear reactor headspace and can react explosively with another gas found in the air forming water. Derive a balanced chemical reaction between the nuclear reactor-generated gas and the air component. Use your derived chemical reaction and specific conditions and material quantities given in the illustration below to calculate the pressure of the gas generated from the reaction between zirconium and steam in atmospheres (atm) units. The gas constant, R, is 0.08206L.atmK1mol1. Round your answer to three significant figures (no scientific notation accepted). Enter numerical values without units Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


