Question
The gas phase reaction A B+C is being carried out in batch reactor with a volume of 1000 dm at 500 K and 5.0
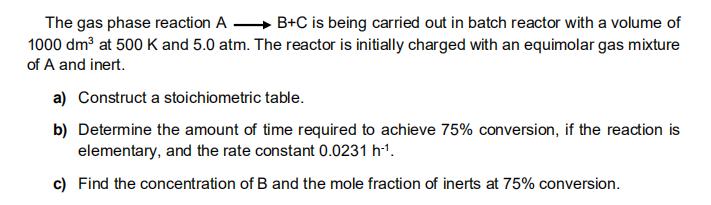
The gas phase reaction A B+C is being carried out in batch reactor with a volume of 1000 dm at 500 K and 5.0 atm. The reactor is initially charged with an equimolar gas mixture of A and inert. a) Construct a stoichiometric table. b) Determine the amount of time required to achieve 75% conversion, if the reaction is elementary, and the rate constant 0.0231 h1. c) Find the concentration of B and the mole fraction of inerts at 75% conversion.
Step by Step Solution
3.51 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Manufacturing Processes for Engineering Materials
Authors: Serope Kalpakjian, Steven Schmid
5th edition
132272717, 978-0132272711
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



