Answered step by step
Verified Expert Solution
Question
1 Approved Answer
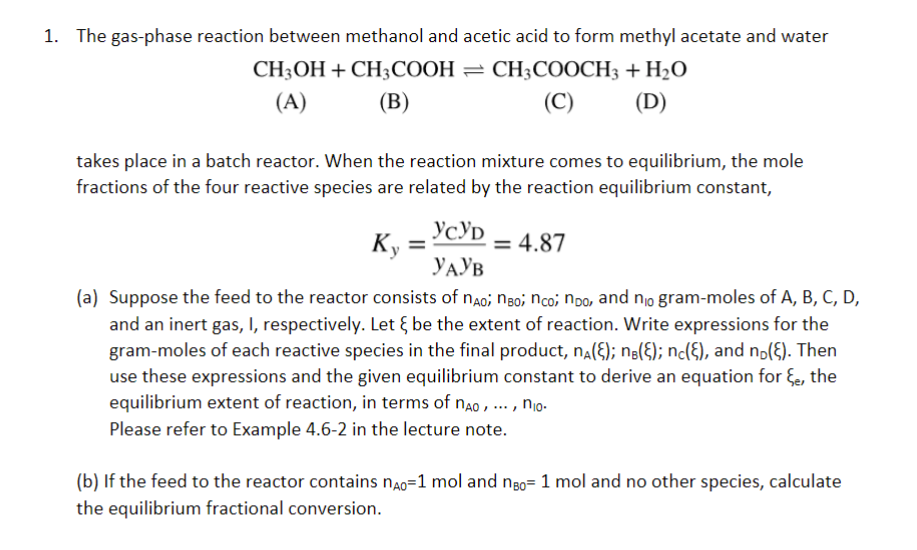
The gas - phase reaction between methanol and acetic acid to form methyl acetate and water C H 3 O H ( A ) +
The gasphase reaction between methanol and acetic acid to form methyl acetate and water
ACOO COO D
A CHOH
B CHCOOH
C CHCOOCH
D HO
takes place in a batch reactor. When the reaction mixture comes to equilibrium, the mole
fractions of the four reactive species are related by the reaction equilibrium constant,
a Suppose the feed to the reactor consists of ;;; and grammoles of
and an inert gas, I, respectively. Let be the extent of reaction. Write expressions for the
grammoles of each reactive species in the final product, ;; and Then
use these expressions and the given equilibrium constant to derive an equation for the
equilibrium extent of reaction, in terms of dots,
Please refer to Example in the lecture note.
b If the feed to the reactor contains mol and mol and no other species, calculate
the equilibrium fractional conversion.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


