Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The gas stream from a chemical reactor contains 25% by mole of ammonia, the remainder being inert gases. The total flow entering an absorption tower
The gas stream from a chemical reactor contains 25% by mole of ammonia, the remainder being inert gases. The total flow entering an absorption tower is 181.4 kmol/h at 303 K and 1.013 x 105 Pa, and the stripping fluid is water containing 0.005 mole fractions of ammonia. The outlet gas concentration contains 2 mol% ammonia. Find the minimum flow (L'min). Plot the balance and operating curves using 1.5 times the minimum value. 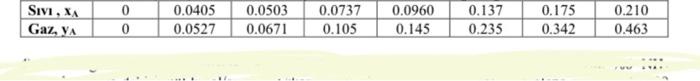
\begin{tabular}{|l|l|l|l|l|l|l|l|l|} \hline Siv1, xA & 0 & 0.0405 & 0.0503 & 0.0737 & 0.0960 & 0.137 & 0.175 & 0.210 \\ \hline Gaz, yA & 0 & 0.0527 & 0.0671 & 0.105 & 0.145 & 0.235 & 0.342 & 0.463 \\ \hline \end{tabular} Equilibrium values of the Ammonia-Water system under process conditions:
upper liquid bottom gas

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


