Answered step by step
Verified Expert Solution
Question
1 Approved Answer
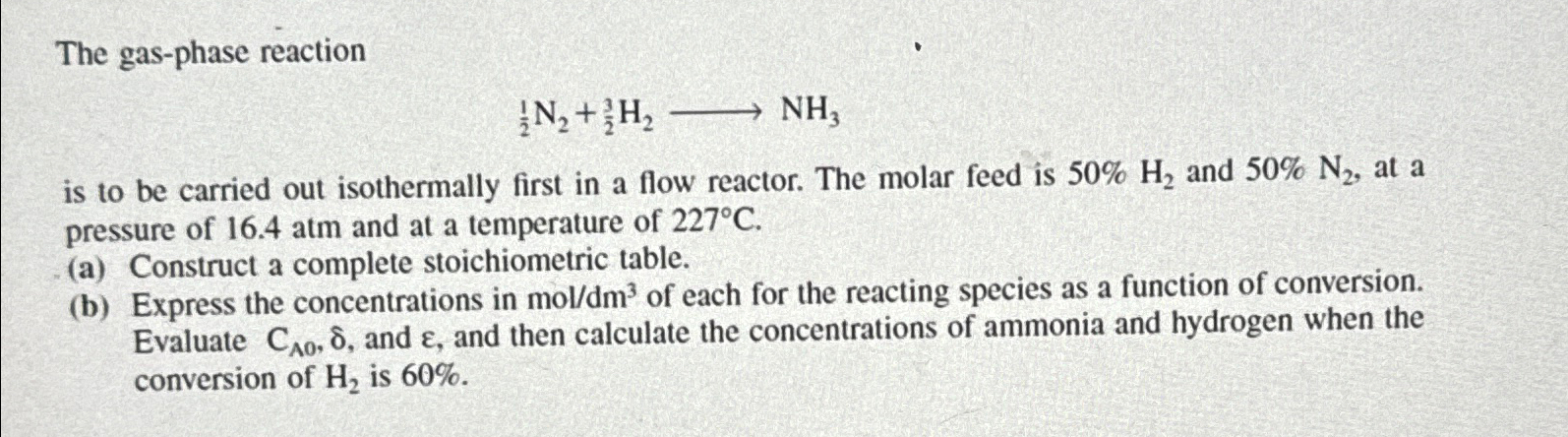
The gas-phase reaction (1)/(2)N_(2)+(3)/(2)H_(2)longrightarrowNH_(3) is to be carried out isothermally first in a flow reactor. The molar feed is 50%H_(2) and 50%N_(2) , at
The gas-phase reaction\
(1)/(2)N_(2)+(3)/(2)H_(2)longrightarrowNH_(3)\ is to be carried out isothermally first in a flow reactor. The molar feed is
50%H_(2)and
50%N_(2), at a pressure of
16.4atmand at a temperature of
227\\\\deg C.\ (a) Construct a complete stoichiometric table.\ (b) Express the concentrations in
mo(l)/(d)m^(3)of each for the reacting species as a function of conversion. Evaluate
C_(A0),\\\\delta , and
\\\\epsi , and then calculate the concentrations of ammonia and hydrogen when the conversion of
H_(2)is
60%.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


