Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The general energy balance in heated tanks can be given by the following equation. MCPdtdT=WCPTi+UA(TbT)WCPT In this equation, M is the mass in the tank,
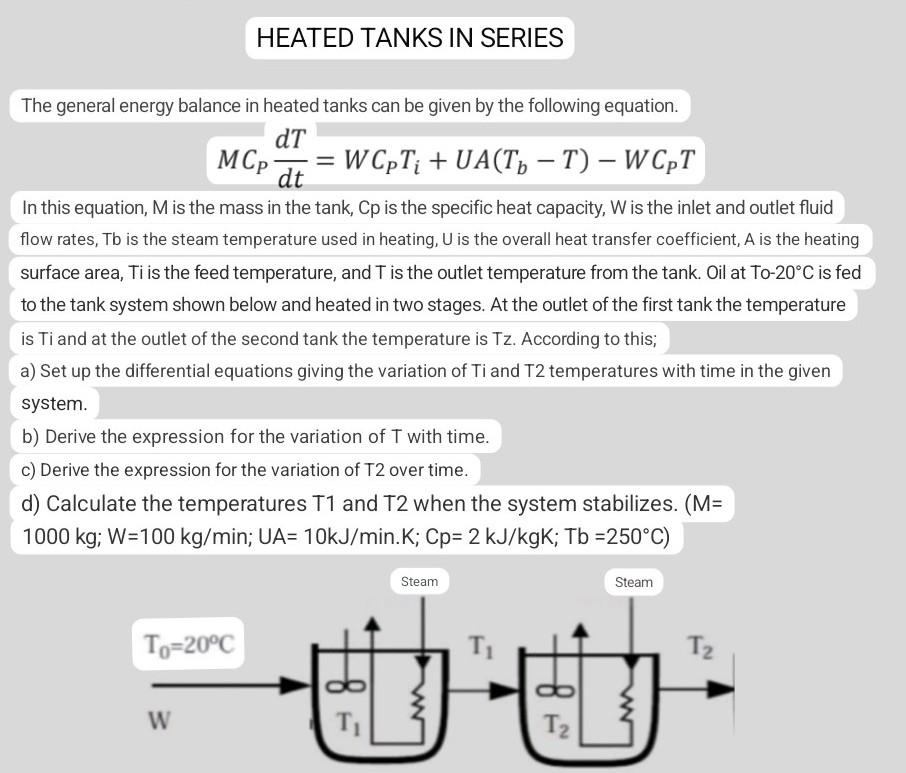
The general energy balance in heated tanks can be given by the following equation. MCPdtdT=WCPTi+UA(TbT)WCPT In this equation, M is the mass in the tank, Cp is the specific heat capacity, W is the inlet and outlet fluid flow rates, Tb is the steam temperature used in heating, U is the overall heat transfer coefficient, A is the heating surface area, Ti is the feed temperature, and T is the outlet temperature from the tank. Oil at To- 20C is fed to the tank system shown below and heated in two stages. At the outlet of the first tank the temperature is Ti and at the outlet of the second tank the temperature is Tz. According to this; a) Set up the differential equations giving the variation of Ti and T2 temperatures with time in the given system. b) Derive the expression for the variation of T with time. c) Derive the expression for the variation of T2 over time. d) Calculate the temperatures T1 and T2 when the system stabilizes. ( M= 1000kg;W=100kg/min;UA=10kJ/min.K;Cp=2kJ/kgK;Tb=250C)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


