Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Gibb's Free Energy (AG) can be used to determine if a reaction is spontaneous as written. It is calculated from two factors, enthalpy
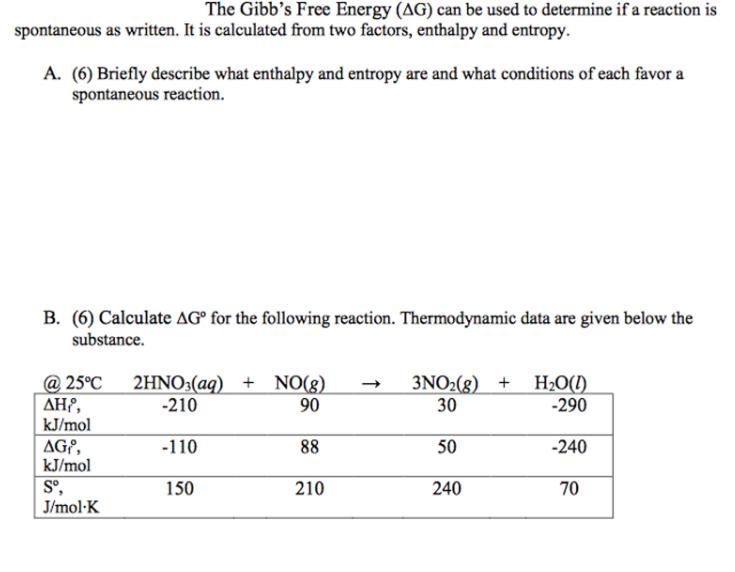
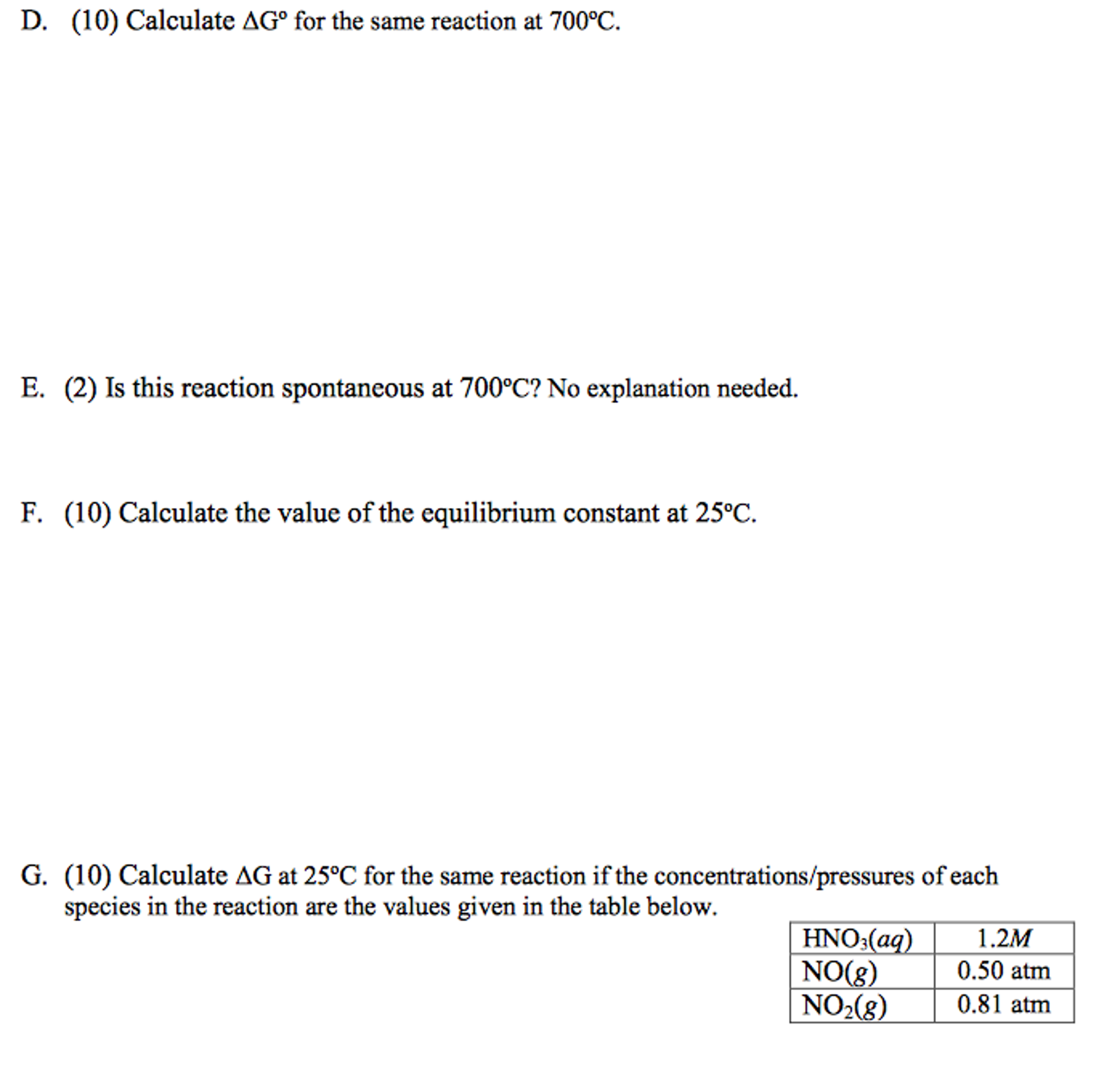
The Gibb's Free Energy (AG) can be used to determine if a reaction is spontaneous as written. It is calculated from two factors, enthalpy and entropy. A. (6) Briefly describe what enthalpy and entropy are and what conditions of each favor a spontaneous reaction. B. (6) Calculate AG for the following reaction. Thermodynamic data are given below the substance. @ 25C 2HNO3(aq) + NO(g) -> 3NO2(g) + H2O(l) , -210 90 30 -290 kJ/mol AG, -110 88 50 -240 kJ/mol S, 150 210 240 70 J/mol-K D. (10) Calculate AG for the same reaction at 700C. E. (2) Is this reaction spontaneous at 700C? No explanation needed. F. (10) Calculate the value of the equilibrium constant at 25C. G. (10) Calculate AG at 25C for the same reaction if the concentrations/pressures of each species in the reaction are the values given in the table below. HNO3(aq) NO(g) 1.2M 0.50 atm NO2(g) 0.81 atm
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer A Step 1 Enthalpy is a measure of the total energy of a thermodynamic system including both i...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


