Answered step by step
Verified Expert Solution
Question
1 Approved Answer
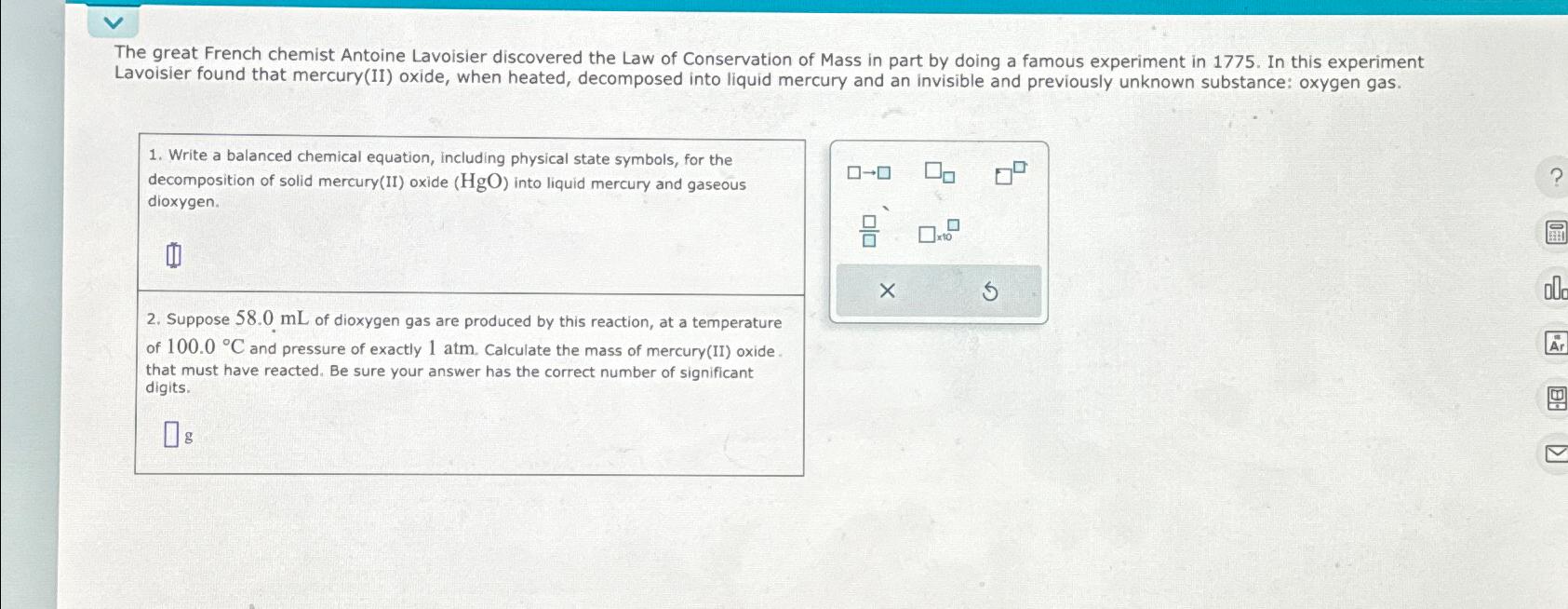
The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in 1 7 7 5
The great French chemist Antoine Lavoisier discovered the Law of Conservation of Mass in part by doing a famous experiment in In this experiment Lavoisier found that mercuryII oxide, when heated, decomposed into liquid mercury and an invisible and previously unknown substance: oxygen gas.
Write a balanced chemical equation, including physical state symbols, for the decomposition of solid mercuryII oxide into liquid mercury and gaseous dioxygen.
Suppose of dioxygen gas are produced by this reaction, at a temperature of and pressure of exactly atm. Calculate the mass of mercuryII oxide. that must have reacted. Be sure your answer has the correct number of significant digits.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


