Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Heisenberg Uncertainty Principle states ?? /2 if [A,B] 0. Show that for the particle in the box in accordance with the uncertainty principle. This
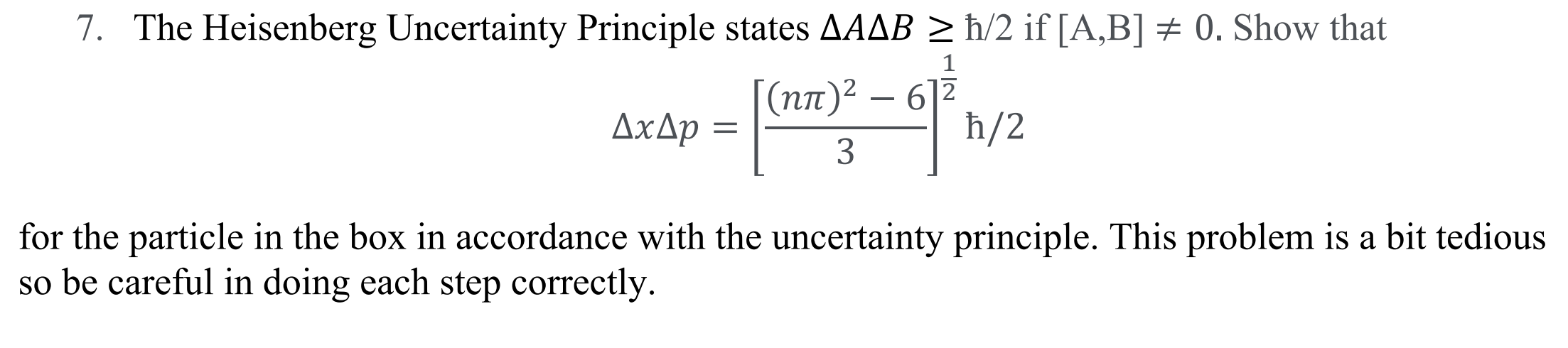
The Heisenberg Uncertainty Principle states
?? /2 if [A,B] 0. Show that
for the particle in the box in accordance with the uncertainty principle. This problem is a bit tedious
so be careful in doing each step correctly.
1 7. The Heisenberg Uncertainty Principle states AAAB > /2 if [A,B] 0. Show that [(nn)2 612 AxAp /2 3 = for the particle in the box in accordance with the uncertainty principle. This problem is a bit tedious so be careful in doing each step correctlyStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


