Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The initial water concentration in the gas was c o = 9 2 6 1 0 - 6 k g water ? k g nitrogen.
The initial water concentration in the gas was water
nitrogen. The breakthrough data are as follows.
A value of is desired at the break point. Do as follows.
a Determine the breakpoint time, the fraction of total capacity used up to
the break point, the length of the unused bed, and the saturation loading
capacity of the solid.
b For a proposed column length calculate the breakpoint
time and fraction of total capacity used.
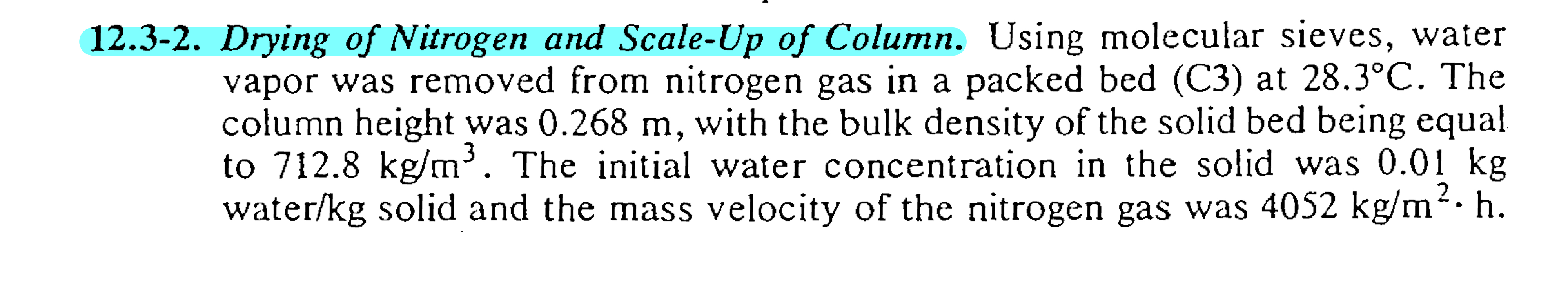
Ans. a fraction used Drying of Nitrogen and ScaleUp of Column. Using molecular sieves, water
vapor was removed from nitrogen gas in a packed bed C at The
column height was with the bulk density of the solid bed being equal
to The initial water concentration in the solid was
water solid and the mass velocity of the nitrogen gas was
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


