Answered step by step
Verified Expert Solution
Question
1 Approved Answer
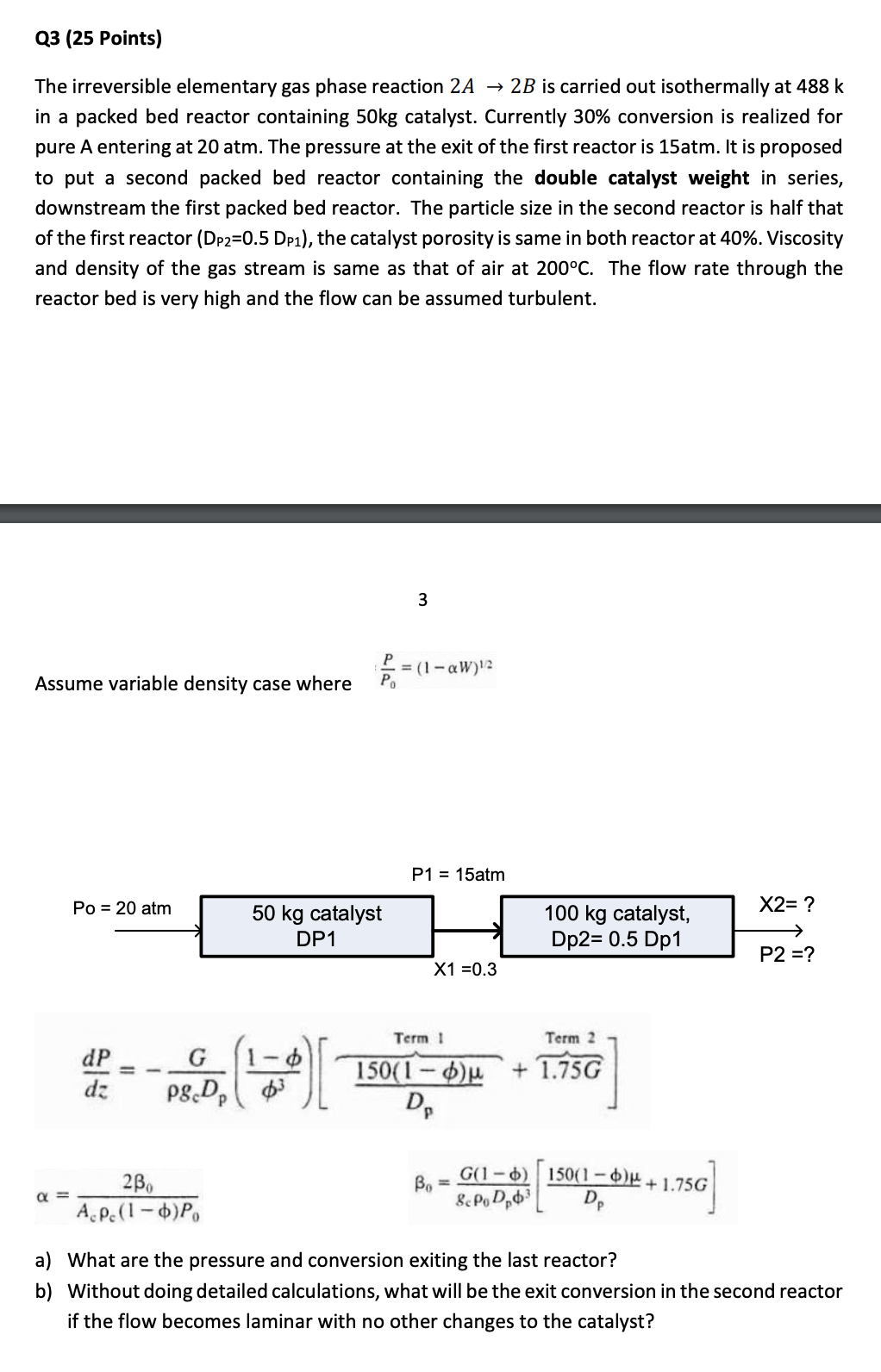
The irreversible elementary gas phase reaction 2 A 2 B is carried out isothermally at 4 8 8 k in a packed bed reactor containing
The irreversible elementary gas phase reaction is carried out isothermally at
in a packed bed reactor containing catalyst. Currently conversion is realized for
pure A entering at atm. The pressure at the exit of the first reactor is atm. It is proposed
to put a second packed bed reactor containing the double catalyst weight in series,
downstream the first packed bed reactor. The particle size in the second reactor is half that
of the first reactor the catalyst porosity is same in both reactor at Viscosity
and density of the gas stream is same as that of air at The flow rate through the
reactor bed is very high and the flow can be assumed turbulent.
Assume variable density case where
a What are the pressure and conversion exiting the last reactor?
b Without doing detailed calculations, what will be the exit conversion in the second reactor
if the flow becomes laminar with no other changes to the catalyst?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


