Answered step by step
Verified Expert Solution
Question
1 Approved Answer
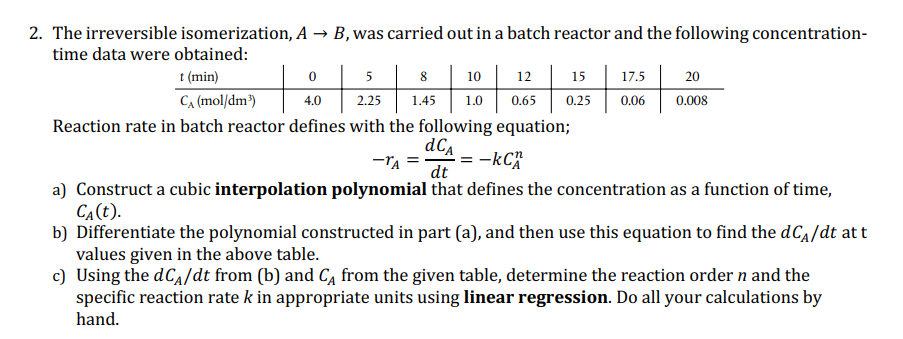
The irreversible isomerization, A B , was carried out in a batch reactor and the following concentration - time data were obtained: Reaction rate in
The irreversible isomerization, was carried out in a batch reactor and the following concentration
time data were obtained:
Reaction rate in batch reactor defines with the following equation;
a Construct a cubic interpolation polynomial that defines the concentration as a function of time,
b Differentiate the polynomial constructed in part a and then use this equation to find the at
values given in the above table.
c Using the from b and from the given table, determine the reaction order and the
specific reaction rate in appropriate units using linear regression. Do all your calculations by
hand. In your hand calculations for Problem use digits arithmetics.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


