Question
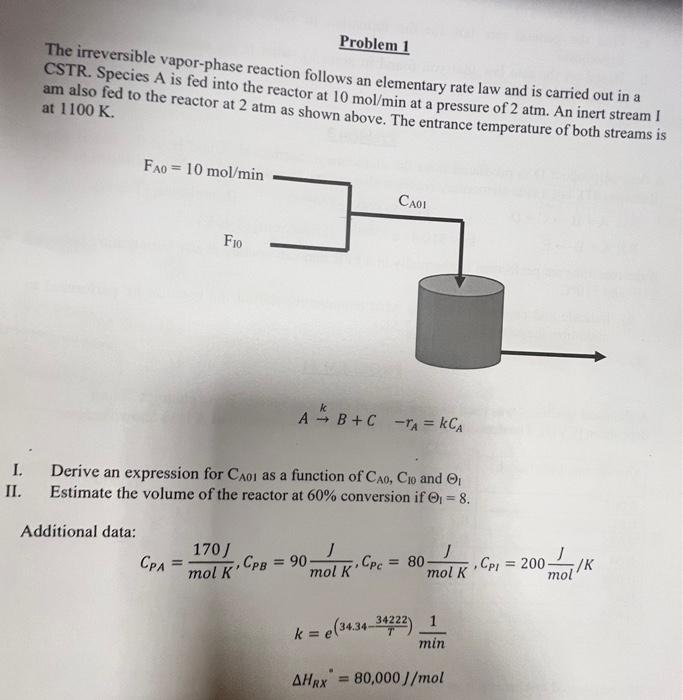
The irreversible vapor-phase reaction follows an elementary rate law and is carried out in a CSTR. Species A is fed into the reactor at 10
The irreversible vapor-phase reaction follows an elementary rate law and is carried out in a CSTR. Species A is fed into the reactor at 10 mol/min at a pressure of 2 atm. An inert stream ! am also fed to the reactor at 2 atm as shown above. The entrance temperature of both streams is at 1100 K.
Fao = 10 mol/min
CAOl
Fio
A K B+ C - TA = KCA
I.
IL.
Derive an expression for CaoI as a function of CAo, Cio and
Estimate the volume of the reactor at 60% conversion if 01 = 8.
Additional data:
170 J
]
]
=
mol K" CPB = 90
mol K" CPc = 80
mol K Cpi = 200 mol/K
M = (3431-34222)
1
min
AHrx
= 80,000 J/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


