Question
A student placed 0.375 mol of PCl3(g) and 0.319 mol of Cl(g) into a 1.00 liter container at 250 C. After the reaction PC13(g)
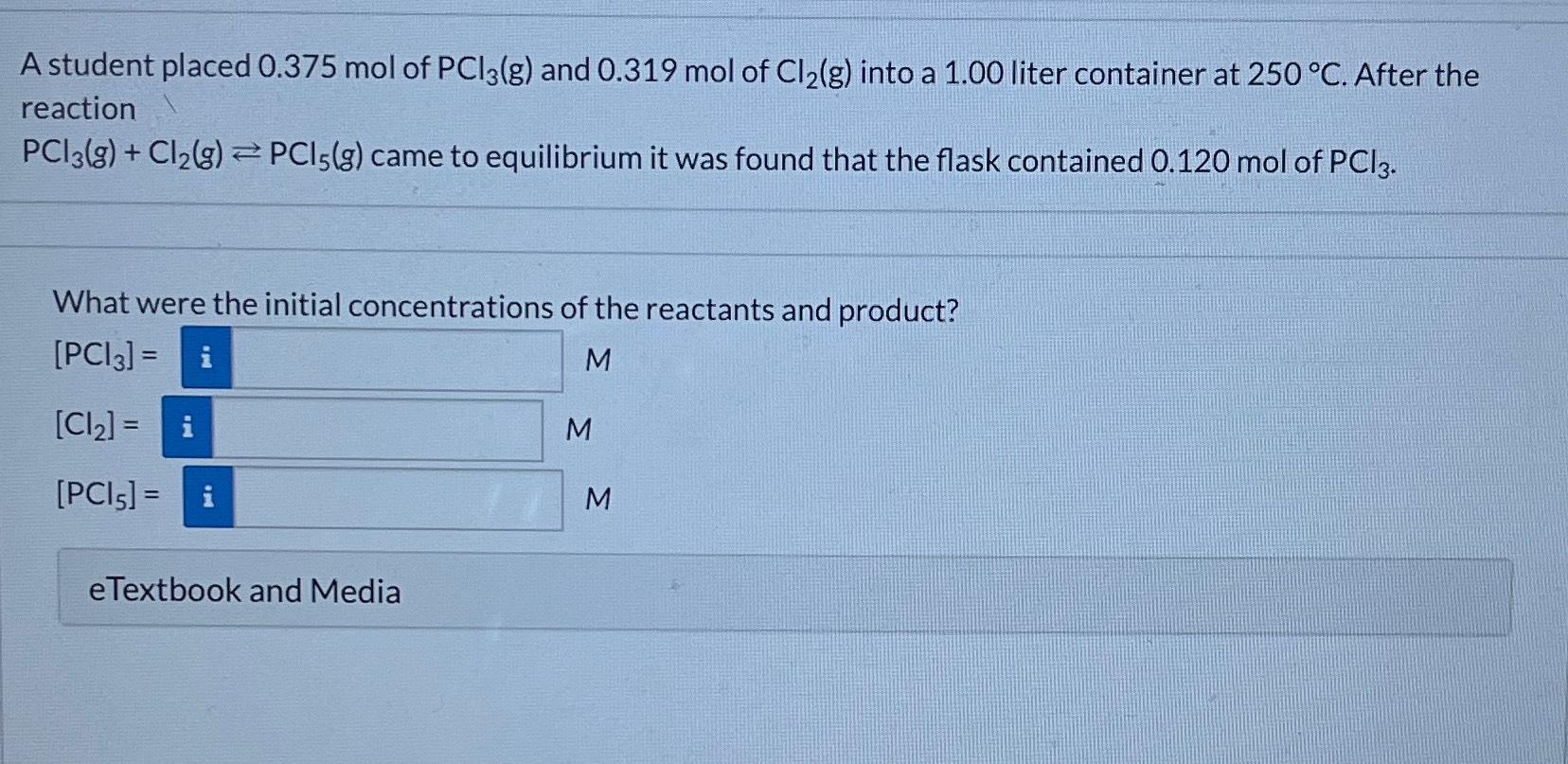
A student placed 0.375 mol of PCl3(g) and 0.319 mol of Cl(g) into a 1.00 liter container at 250 C. After the reaction PC13(g) + Cl(g) PCI5(g) came to equilibrium it was found that the flask contained 0.120 mol of PCI 3. What were the initial concentrations of the reactants and product? [PCI3] = i M [Cl] = i [PCI5] = i eTextbook and Media M M
Step by Step Solution
3.33 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
To solve this problem we can use the information provided and the concept of equilibrium concentrati...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial accounting
Authors: Walter T. Harrison, Charles T. Horngren, William Bill Thomas
8th Edition
9780135114933, 136108865, 978-0136108863
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



