Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Vermilion is synthetic mercury(II) sulfide and that it is very toxic because of its mercury. John has a beautiful red-colored wooden bowl which he
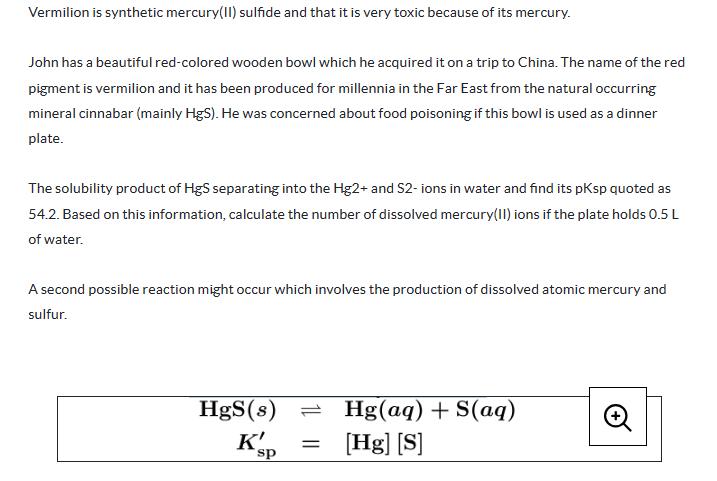
Vermilion is synthetic mercury(II) sulfide and that it is very toxic because of its mercury. John has a beautiful red-colored wooden bowl which he acquired it on a trip to China. The name of the red pigment is vermilion and it has been produced for millennia in the Far East from the natural occurring mineral cinnabar (mainly HgS). He was concerned about food poisoning if this bowl is used as a dinner plate. The solubility product of HgS separating into the Hg2+ and S2- ions in water and find its pKsp quoted as 54.2. Based on this information, calculate the number of dissolved mercury(II) ions if the plate holds 0.5 L of water. A second possible reaction might occur which involves the production of dissolved atomic mercury and sulfur. HgS(s) K's p sp Hg(aq) + S(aq) = [Hg] [S]
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Based on the information provided the solubility product Ksp of HgS is given as 542 To calculat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


