Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The kinetics of the gas phase reaction between sulfur tetrafluoride (SF4) and trifluoromethyl hypofluorite (CF3OF) was studied in a constant volume reactor. The primary reaction
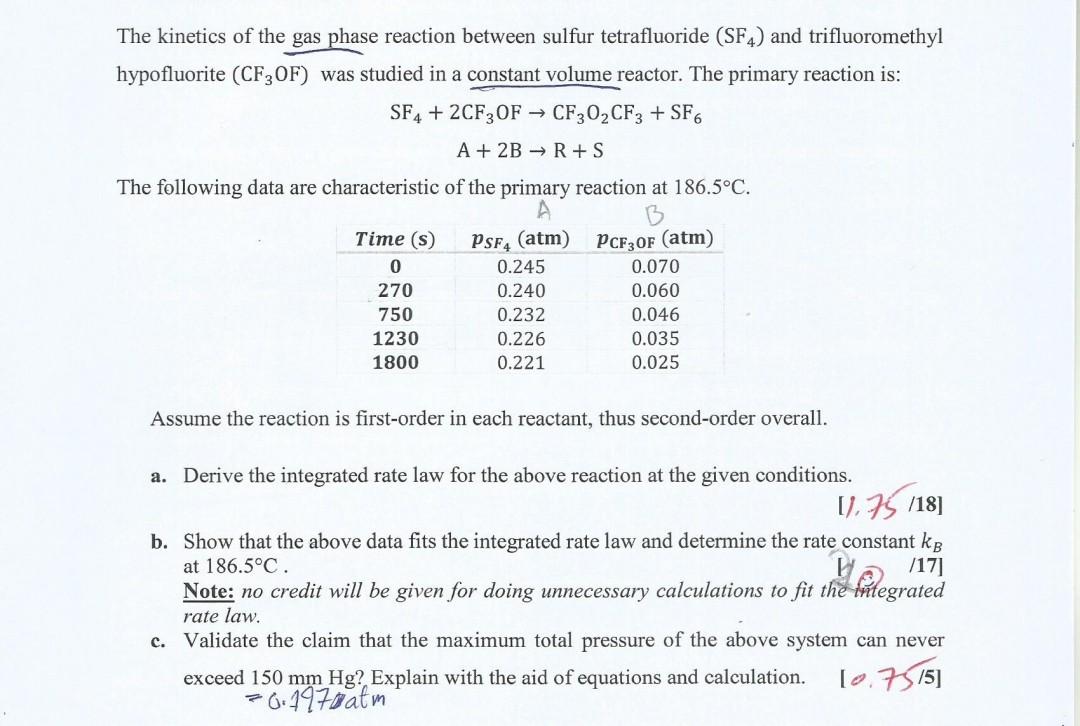
The kinetics of the gas phase reaction between sulfur tetrafluoride (SF4) and trifluoromethyl hypofluorite (CF3OF) was studied in a constant volume reactor. The primary reaction is: SF4+2CF3OFA+2BCF3O2CF3+SF6R+S The following data are characteristic of the primary reaction at 186.5C. Assume the reaction is first-order in each reactant, thus second-order overall. a. Derive the integrated rate law for the above reaction at the given conditions. [1,75118] b. Show that the above data fits the integrated rate law and determine the rate constant kB at 186.5C. Note: no credit will be given for doing unnecessary calculations to fit the integrated rate law. c. Validate the claim that the maximum total pressure of the above system can never exceed 150mmHg ? Explain with the aid of equations and calculation. [0.73/5] =0.197 atm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


