Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The last questions answer is either a 1 or 2 At times, it is not clear how many significant digits are present for a number
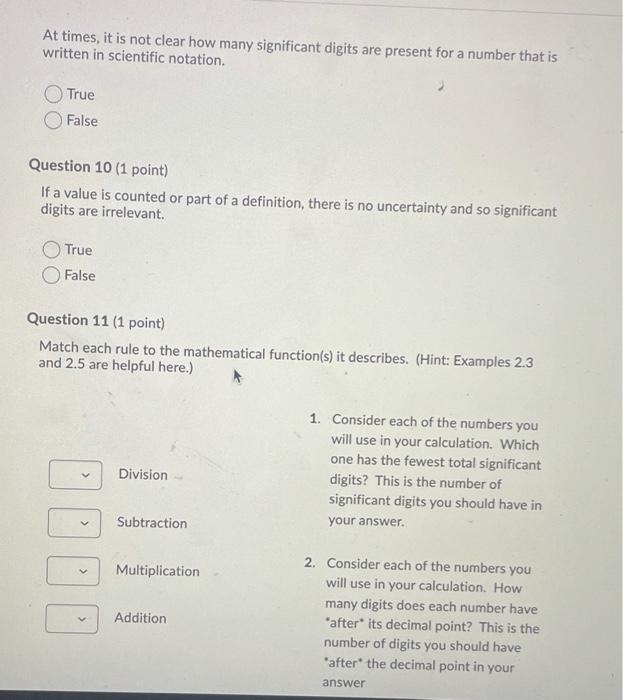
The last questions answer is either a 1 or 2
At times, it is not clear how many significant digits are present for a number that is written in scientific notation. True False Question 10 (1 point) If a value is counted or part of a definition, there is no uncertainty and so significant digits are irrelevant. True False Question 11 (1 point) Match each rule to the mathematical function(s) it describes. (Hint: Examples 2.3 and 2.5 are helpful here.) Division DOO 1. Consider each of the numbers you will use in your calculation. Which one has the fewest total significant digits? This is the number of significant digits you should have in your answer. Subtraction Multiplication Addition 2. Consider each of the numbers you will use in your calculation. How many digits does each number have "after its decimal point? This is the number of digits you should have "after the decimal point in your Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


