Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the last two photos are the pages with the answers thats need to be completed please. Penny For Your Thoughts Introduction The first people to
the last two photos are the pages with the answers thats need to be completed please. 


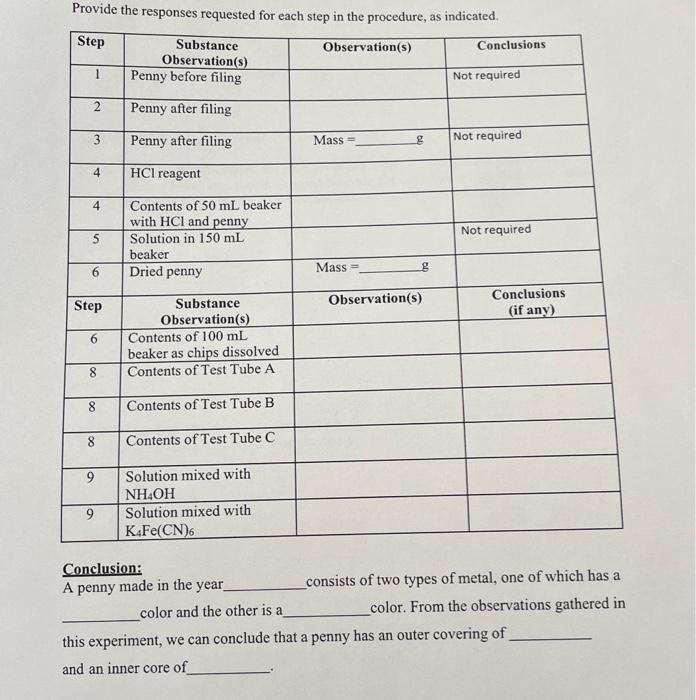

Penny For Your Thoughts Introduction The first people to scientifically study nature had no analytical balances, graduated cylinders, or other accurate measuring devices by which they could colleet data. Those pioneers had only their five senses and a deep desire to understand the universe around them. Even without sophisticated instruments, however, much was discovered, and today. good scientists still use their five senses to make observations which lead to great advances in understanding the world in which we live. This laboratory experiment will give you experience observing some of the chemical and physical properties of a common object. From your observations, you will be able to draw significant conclusions about the composition of the object. Before you can truly be successful in this experiment, you must clearly understand the difference between an observation and a conclusion. The two really are quite different things, although they are sometimes expressed in very similar ways. To a scientist, an observation is a record of any event or property that can be detected by either the five senses or a scientific instrument. A conclusion is a judgment about the meaning of one or more observations. Observations provide the justification for conclusions that are drawn, and a reasonable conclusion can come about only by intelligent interpretation of one or more observations. As you perform this week's experiment, try to identify the conclusions that you make, and try to determine what it is about the conclusions that distinguished them from your observations. Experimental Procedure 1. Bring to lab an American penny minted after 1982. Observe the penny. Is it solid, liquid, or gas? For a solid, you can ask more questions: Is it hard or soft? Is it dull or shiny? Is it brittle or flexible? What is its color? 2. Using a triangular file, mark the edge of the penny by firmly drawing the file against the edge of the penny at approximately a 45 angle. Consider the face of the penny to represent a clock. The first mark is at twelve o'clock. On the same side of the penny, make a second mark at six o'clock. Turn the penny over and make two additional marks at the three and nine o'clock positions. Now observe the penny. What color do you observe at the file marks? What can you conclude about the composition (makeup) of the penny? It should be silvery. Color, as we know, is an intrinsic property. Hence, we can conclude (not observe!) that a penny consists of two different metals. One metal is brown and the other is silver in color. 3. Weigh the penny on an analytical balance. Record the mass in your notebook. 4. Place the penny in a clean 50mL beaker. In one of the fume hoods, add 10.0mL of concentrited HCl (hydrochloric acid) to the beaker. (CAUTION: Acids and bases of dangerous reagents, especially in concentated form. If you spill a small quantity and mopping the area with the paper towely by spills of 50mL or more should be immediately reported to the instructor. Always, wash these reagents of of your skin immediately with lots of tap water. Do not work with these reagents unless you are wearing safety goggles. What can you say about the HCl reagent? Is it a solid, liquid, of gas? What is its. color? Is the solution clear or eloudy? Does it have an edor? (CAUTION: peser place a chemical directly under your nose to smell it. Instead, hold the chemical a few inches in front of you with one hand, and gently wave the fumes toward your nose with your other hand.) Now look at the beaker and its contents. Would the bubbles be classified as solid, liquid, or gas? Did the HCl originally appear to have these bubbles? A chemist could perform tests that would show that the gas is hydrogen, and that it has the chemical formula, H2. Allow your penny to produce hydrogen for 20 minutes before you proceed to the next step. 5. Add 30mL of distilled water to the beaker holding the penny and HCl. Transfer the solution (leave the penny behind) to a clean 150mL beaker, and add another 30mL of distilled water to the solution. Save the solution for Step 8. Make some observations on this solution. 6. Rinse the penny in tap water to remove all of the acid. Dry the penny and weigh it on an analytical balance. Record the mass. How much metal was dissolved by the acid? The outer surface of the penny should be very flexible at each point where it was filed. Break off this outer layer and place these brown metal chips into a clean 100 mL beaker. In a fume hood add 10 to 15 drops of concentrated HNO3 (nitric acid) to the beaker. Try to dissolve as many of the brown chips as possible. Wait 5 minutes and then add 20mL distilled water. Save this solution for Step 9. 7. In following steps you will need to measure 0.5mL. So, take a dropper and measure how many drops it takes to reach the 1-mL line on a 10mL graduated cylinder. Use half this number of drops to measure the 0.5mL of solution in the steps below. 8. Place 0.5mL of the solution from Step 5 (the solution in the 150mL beaker) into each of three 1075mm test tubes. Label the tubes A, B, and C. Test Tube A: Add 0.5mL of 1MNH4C2H3O2, ammonium acetate solution*, followed by 0.5mL of 0.2MK2CrO4, potassium chromate solution, to the test tube. Record your observations. Formation of a yellow solid (a precipitate) would allow us to conclude that the metal in the penny that reacts with the HCl is lead ( Pb). Test Tube B: Add dilute (5M)NH4OH (ammonium hydroxide) until the solution is basic. You can tell if the solution is basic by tentiong hroxide) until the solution is Litmus Test: Wet the red litmus paper with distilled water. Dip your glass rod spatula or glass rod. A blue color Touch the wet litmus paper with the tip of the If the solution is not basic, appears if the solution is basic. After the solution is not basic, add 5 more drops of NH4OH and test again. After the solution turns basic, add 24 drops of dimethylglyoxime solution to the test tube. The formation of a strawberry-red precipitate would allow us to conclude that the interior metal of the penny is nickel. Record your observations. Test Tube C: Add 34 drops of K4Fe(CN)6 (potassium ferrocyanide) solution to the test tube. The formation of a grayish-white to bluish-green precipitate would indicate that the penny's interior metal is zinc. Record your observations. Can we now conclude which metal reacted with the HCl and, therefore, which metal made up the interior of the penny? * "1 M " is an abbreviation for "one molar," a measure of the concentration of the reagent. 9. To half of the solution from Step 6(100mL beaker), add concentrated (15M) NH4OH until the solution is basic. Ignoring any undissolved metal chips, record your observations. Any copper present will react with the NH4OH to form a blue solution. To the other half of the solution, add 24 drops of K4Fe(CN) s solution. The formation of a red to reddish-brown precipitate the presence of copper metal in solution. Record your observations. 10. Dispose of your solutions and clean-up as follows. For the solution from Step 6 in your 150mL beaker, add a small amount of solid sodium bicarbonate and observed formation of bubbles of carbon dioxide. Continue adding sodium bicarbonate, stirring the solution with a glass stirring rod and testing the solution with pH paper until no bubbles are generated and the pH of the solution is no longer acidic. The solution can now be poured into a running stream of water down the sink. Wash all of your other solutions down the sink drains with large amounts of water. Use a test tube brush to remove any precipitate from your test tubes. Return the cleaned glassware to your drawer. Provide the responses requested for each step in the procedure, as indicated. Conclusion: A penny made in the year consists of two types of metal, one of which has a color and the other is a color. From the observations gathered in this experiment, we can conclude that a penny has an outer covering of and an inner core of 1. Comment on any errors that may have occurred during the experiment. How could these errors have affected your results? Answer the following questions: 2. In step 9 of the procedure, the solution made in step 6 was divided into two portions, and each portion was tested. Suggest a reason for performing step 9 this way, rather than doing a single test on all of the solution produced in step 6 . 3. Is a medical diagnosis an observation or a conclusion? (Be sure that you support your answer with clear, concise reasons.) Penny For Your Thoughts Introduction The first people to scientifically study nature had no analytical balances, graduated cylinders, or other accurate measuring devices by which they could colleet data. Those pioneers had only their five senses and a deep desire to understand the universe around them. Even without sophisticated instruments, however, much was discovered, and today. good scientists still use their five senses to make observations which lead to great advances in understanding the world in which we live. This laboratory experiment will give you experience observing some of the chemical and physical properties of a common object. From your observations, you will be able to draw significant conclusions about the composition of the object. Before you can truly be successful in this experiment, you must clearly understand the difference between an observation and a conclusion. The two really are quite different things, although they are sometimes expressed in very similar ways. To a scientist, an observation is a record of any event or property that can be detected by either the five senses or a scientific instrument. A conclusion is a judgment about the meaning of one or more observations. Observations provide the justification for conclusions that are drawn, and a reasonable conclusion can come about only by intelligent interpretation of one or more observations. As you perform this week's experiment, try to identify the conclusions that you make, and try to determine what it is about the conclusions that distinguished them from your observations. Experimental Procedure 1. Bring to lab an American penny minted after 1982. Observe the penny. Is it solid, liquid, or gas? For a solid, you can ask more questions: Is it hard or soft? Is it dull or shiny? Is it brittle or flexible? What is its color? 2. Using a triangular file, mark the edge of the penny by firmly drawing the file against the edge of the penny at approximately a 45 angle. Consider the face of the penny to represent a clock. The first mark is at twelve o'clock. On the same side of the penny, make a second mark at six o'clock. Turn the penny over and make two additional marks at the three and nine o'clock positions. Now observe the penny. What color do you observe at the file marks? What can you conclude about the composition (makeup) of the penny? It should be silvery. Color, as we know, is an intrinsic property. Hence, we can conclude (not observe!) that a penny consists of two different metals. One metal is brown and the other is silver in color. 3. Weigh the penny on an analytical balance. Record the mass in your notebook. 4. Place the penny in a clean 50mL beaker. In one of the fume hoods, add 10.0mL of concentrited HCl (hydrochloric acid) to the beaker. (CAUTION: Acids and bases of dangerous reagents, especially in concentated form. If you spill a small quantity and mopping the area with the paper towely by spills of 50mL or more should be immediately reported to the instructor. Always, wash these reagents of of your skin immediately with lots of tap water. Do not work with these reagents unless you are wearing safety goggles. What can you say about the HCl reagent? Is it a solid, liquid, of gas? What is its. color? Is the solution clear or eloudy? Does it have an edor? (CAUTION: peser place a chemical directly under your nose to smell it. Instead, hold the chemical a few inches in front of you with one hand, and gently wave the fumes toward your nose with your other hand.) Now look at the beaker and its contents. Would the bubbles be classified as solid, liquid, or gas? Did the HCl originally appear to have these bubbles? A chemist could perform tests that would show that the gas is hydrogen, and that it has the chemical formula, H2. Allow your penny to produce hydrogen for 20 minutes before you proceed to the next step. 5. Add 30mL of distilled water to the beaker holding the penny and HCl. Transfer the solution (leave the penny behind) to a clean 150mL beaker, and add another 30mL of distilled water to the solution. Save the solution for Step 8. Make some observations on this solution. 6. Rinse the penny in tap water to remove all of the acid. Dry the penny and weigh it on an analytical balance. Record the mass. How much metal was dissolved by the acid? The outer surface of the penny should be very flexible at each point where it was filed. Break off this outer layer and place these brown metal chips into a clean 100 mL beaker. In a fume hood add 10 to 15 drops of concentrated HNO3 (nitric acid) to the beaker. Try to dissolve as many of the brown chips as possible. Wait 5 minutes and then add 20mL distilled water. Save this solution for Step 9. 7. In following steps you will need to measure 0.5mL. So, take a dropper and measure how many drops it takes to reach the 1-mL line on a 10mL graduated cylinder. Use half this number of drops to measure the 0.5mL of solution in the steps below. 8. Place 0.5mL of the solution from Step 5 (the solution in the 150mL beaker) into each of three 1075mm test tubes. Label the tubes A, B, and C. Test Tube A: Add 0.5mL of 1MNH4C2H3O2, ammonium acetate solution*, followed by 0.5mL of 0.2MK2CrO4, potassium chromate solution, to the test tube. Record your observations. Formation of a yellow solid (a precipitate) would allow us to conclude that the metal in the penny that reacts with the HCl is lead ( Pb). Test Tube B: Add dilute (5M)NH4OH (ammonium hydroxide) until the solution is basic. You can tell if the solution is basic by tentiong hroxide) until the solution is Litmus Test: Wet the red litmus paper with distilled water. Dip your glass rod spatula or glass rod. A blue color Touch the wet litmus paper with the tip of the If the solution is not basic, appears if the solution is basic. After the solution is not basic, add 5 more drops of NH4OH and test again. After the solution turns basic, add 24 drops of dimethylglyoxime solution to the test tube. The formation of a strawberry-red precipitate would allow us to conclude that the interior metal of the penny is nickel. Record your observations. Test Tube C: Add 34 drops of K4Fe(CN)6 (potassium ferrocyanide) solution to the test tube. The formation of a grayish-white to bluish-green precipitate would indicate that the penny's interior metal is zinc. Record your observations. Can we now conclude which metal reacted with the HCl and, therefore, which metal made up the interior of the penny? * "1 M " is an abbreviation for "one molar," a measure of the concentration of the reagent. 9. To half of the solution from Step 6(100mL beaker), add concentrated (15M) NH4OH until the solution is basic. Ignoring any undissolved metal chips, record your observations. Any copper present will react with the NH4OH to form a blue solution. To the other half of the solution, add 24 drops of K4Fe(CN) s solution. The formation of a red to reddish-brown precipitate the presence of copper metal in solution. Record your observations. 10. Dispose of your solutions and clean-up as follows. For the solution from Step 6 in your 150mL beaker, add a small amount of solid sodium bicarbonate and observed formation of bubbles of carbon dioxide. Continue adding sodium bicarbonate, stirring the solution with a glass stirring rod and testing the solution with pH paper until no bubbles are generated and the pH of the solution is no longer acidic. The solution can now be poured into a running stream of water down the sink. Wash all of your other solutions down the sink drains with large amounts of water. Use a test tube brush to remove any precipitate from your test tubes. Return the cleaned glassware to your drawer. Provide the responses requested for each step in the procedure, as indicated. Conclusion: A penny made in the year consists of two types of metal, one of which has a color and the other is a color. From the observations gathered in this experiment, we can conclude that a penny has an outer covering of and an inner core of 1. Comment on any errors that may have occurred during the experiment. How could these errors have affected your results? Answer the following questions: 2. In step 9 of the procedure, the solution made in step 6 was divided into two portions, and each portion was tested. Suggest a reason for performing step 9 this way, rather than doing a single test on all of the solution produced in step 6 . 3. Is a medical diagnosis an observation or a conclusion? (Be sure that you support your answer with clear, concise reasons.) 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


