Answered step by step
Verified Expert Solution
Question
1 Approved Answer
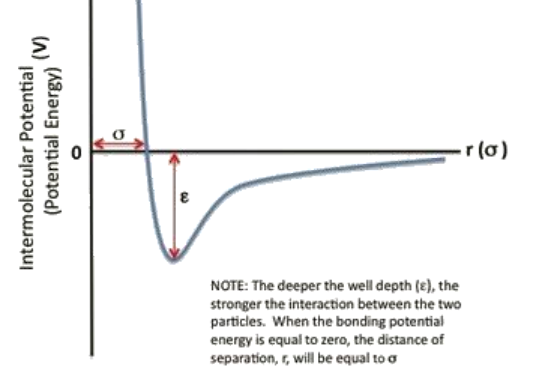
The Lennard - Jones potential model consists of two 'parts'; a steep repulsive term ( 1 / r ^ 1 2 ) , and smoother
The LennardJones potential model consists of two 'parts'; a steep repulsive term r and smoother attractive term r representing van der Waals dispersion forces present in all molecules.
The LennardJones model has found widespread use due to its computational expediency. The LennardJones potential equation:
Vrsigma rsigma r
where,
V is the intermolecular potential between the two atoms or molecules.
is the well depth and a measure of how strongly the two particles attract each other.
sigma is the distance at which the intermolecular potential between the two particles is zero Figure provided
sigma gives a measurement of how close two nonbonding particles can get and is thus referred to as the van der Waals radius. It is equal to onehalf of the internuclear distance between nonbonding particles.
r is the distance of separation between both particles measured from the center of one particle to the center of the other particle
The actual force of interaction along a linear direction is given by:
FrdVr dr
A For the given model, at what distance rmin does the minimum occur for interaction potential, Vr and force Fr
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


